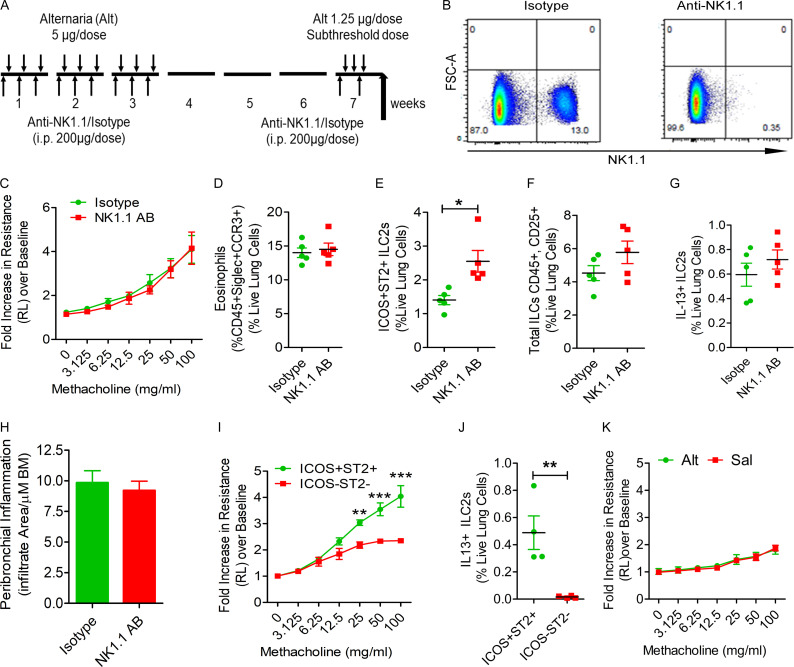
Figure 2.
The role of NK cells and ILCs in memory-driven asthma. (A–C) Role of NK cells and ILCs in memory-driven asthma. (A) A schematic diagram of the timeline of antibody and allergen exposure and recall challenge experiments. An aliquot of 200 µg of an anti-NK1.1 antibody was administered i.p. to Rag1−/− female mice 1 d before each Alt dose during the exposure phase, 1 d before the recall challenges, and 1 h before the last recall challenge. (B–D) A representative flow cytogram (B) for NK cells in the lung digest in NK1.1 antibody– and control antibody–treated mice, airway hyperreactivity (C) and lung eosinophils (CD45+SiglecF+ CCR3+ cells; D). (E–H) Lung ILC2s (CD45+NK1.1−CD25+ICOS+ST2+ cells; E), total ILCs (CD45+CD25+ ILCs; F), IL13+ ILC2s (CD45+CD25+ICOS+ST2+ cells; G) and airway inflammation (H) in the memory model after depletion of NK cells. *, P < 0.01, n = 5, unpaired t test. FSC, forward scatter. (I and J) Induction of asthma by adoptive transfer of memory ILCs. CD45+NK1.1−CD25+ICOS+ST2+ (ICOS+ST2+) and CD45+NK1.1−CD25+ICOS−ST2− (double-negative) ILCs were obtained from Alt/Alt-treated mice. 50,000 cells were intravenously transferred to Rag2−/−:γc−/− mice. 1 d after the transfer, the recipient mice were challenged with Alt for three consecutive days and examined for airway hyperreactivity (lung resistance in response to methacholine) and IL13+ ILC2s 24 h later. **, P < 0.001; ***, P < 0.0001 (two-way ANOVA and unpaired t test, n = 4/group). (K) Naive Rag2−/−:γc−/− mice were challenged with Alt or Sal for three consecutive days and examined for airway hyperreactivity (lung resistance in response to methacholine). Two-way ANOVA, n = 5/group.