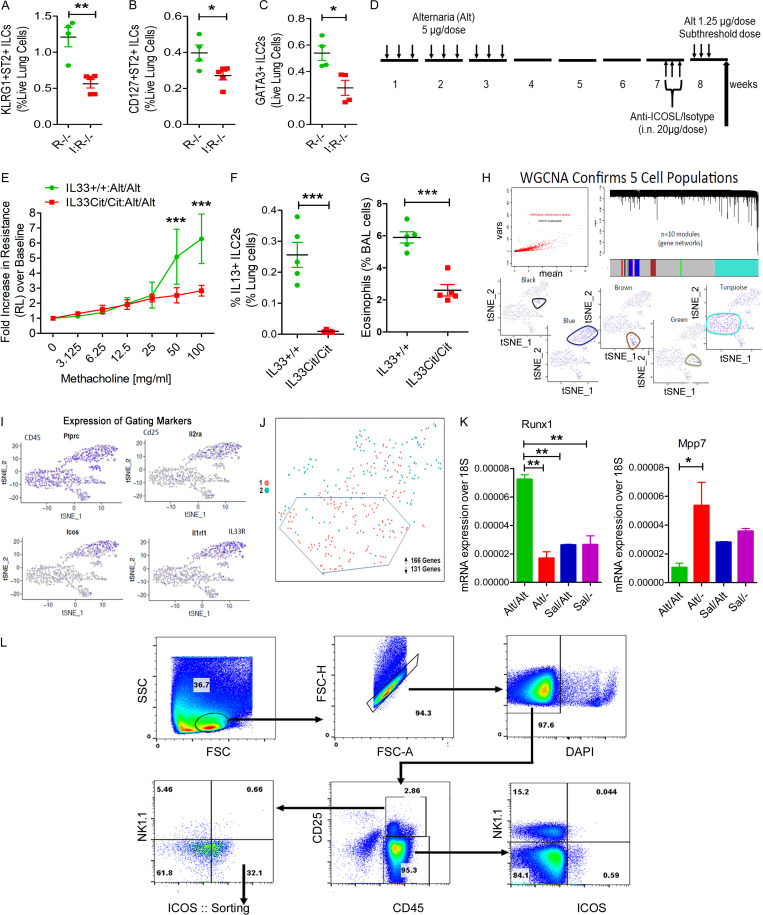
Figure S3.
Role of ICOS and IL33 in memory and gating strategy for scRNA-seq. (A–C) Importance of ICOS for memory-associated asthma. ICOS−/−:Rag1−/− (I:R−/−) and Rag1−/− (R−/−) mice were subjected to the Alt-induced memory-driven asthma protocol as per Fig. 1 A. KLRG1+ST2+, CD127+ ST2+, and GATA3+ ILC2s (CD45+NK1.1−CD25+) cells were measured by FCM. *, P < 0.01; **, P < 0.001; unpaired t test, n = 4/group. (D) A schematic diagram of the timeline of anti-ICOSL/isotype antibody exposure. (E–G) Role of IL33 in memory formation. IL33Cit/Cit and IL33+/+ mice were subjected to the Alt/Alt or Sal/Sal exposure and recall protocol as per Fig. 1 A. Airway hyperreactivity (E), lung IL13+ ILC2s (Lin−CD45+CD25+NK1.1−ICOS+ST2+; F), and BAL eosinophils (G) were measured. ***, P < 0.0001, n = 5/group, unpaired t test. (H) scRNA-seq of lung CD45+ cells. Lung CD45+ cells were sorted following digestion of blood-depleted lungs with collagenase. Cells from each sample were subjected to scRNA-seq by Wafergen iCell8 protocol followed by Illumina Hi-seq sequencing. t-SNE coordinate clustering of cells with WGCNA network module eigengene expression overlaid for modules highly expressed in each particular cluster. Based on genes contained in the cell discriminatory modules, we defined the clusters as macrophages (turquoise), NK cells (black), dendritic cells (green), endothelial cells (brown), and ILC2s (blue). (I) The expression profile of CD45, Il2ra, Icos, and il1rl1 in each cell cluster is shown. (J) Partial separation of memory ILCs (Alt/− and Alt/Alt in red) from control ILCs (Sal/− and Sal/Alt in turquoise) by t-SNE analysis. A total of 161 and 136 genes were up- and down-regulated, respectively, in memory ILCs. (K) Expression of mRNA for Runx1 and Mpp7 in ILCs from the memory models. *, P < 0.01; **, P < 0.001, n = 5/group, one-way ANOVA. (L) The gating and sorting strategy for isolation of CD45+NK1.1−CD25+ICOS+ cells for ATAC-seq. FSC, forward scatter; SSC, side scatter.