Figure 1.
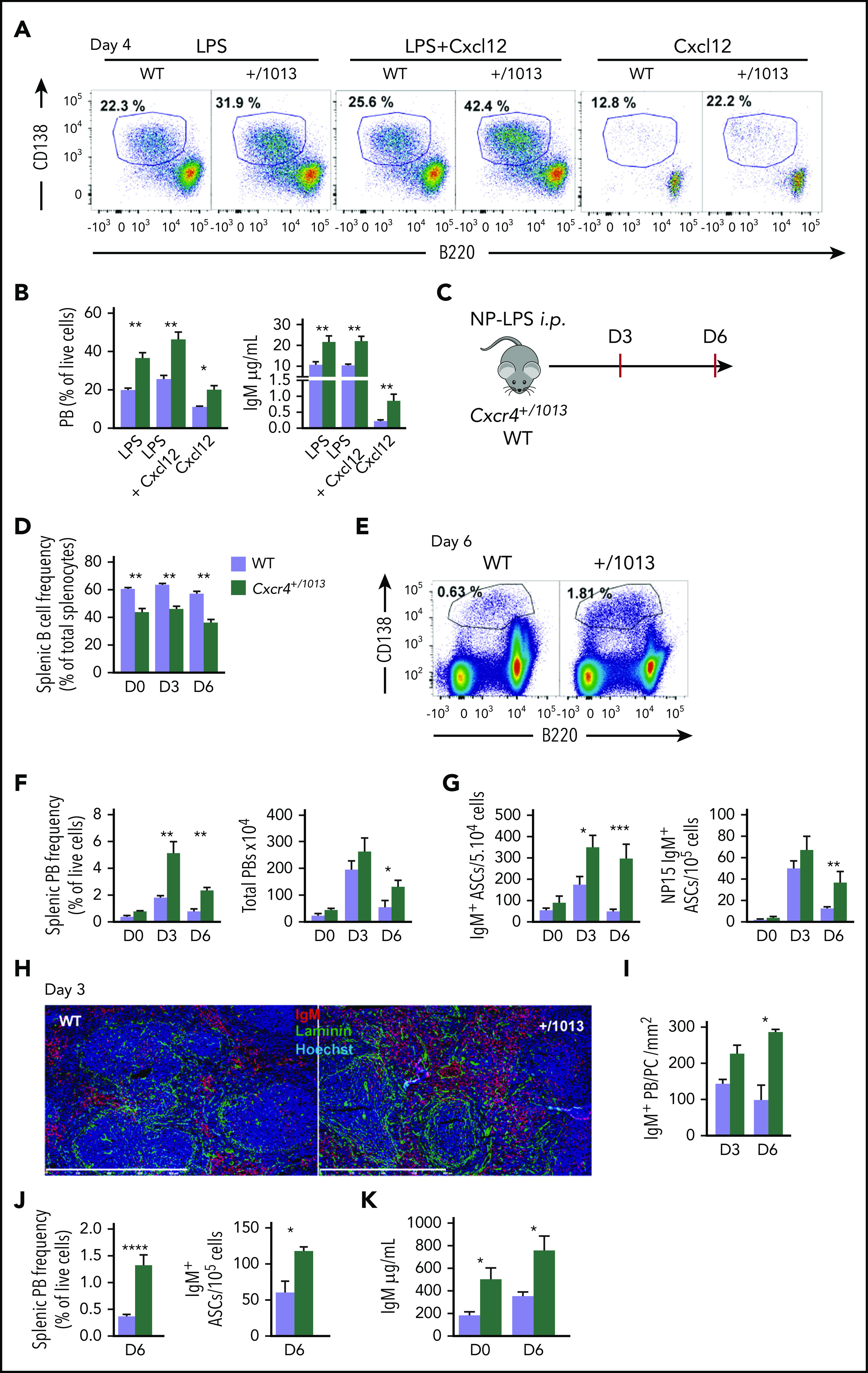
Cxcr4 desensitization limits plasma cell differentiation in vitro and in vivo. (A-B) Splenic B cells were cultured in presence of LPS, LPS+Cxcl12, or Cxcl12 alone for 4 days. (A) Representative dot plots for the gating of PBs (B220loCD138+) generated in vitro after 4 days of culture. (B) The proportion of PBs was determined by FACS and IgM concentrations in the supernatants were determined by ELISA. (C) Schematic diagram for the NP-LPS immunization. (D) Frequency of splenic B cells (CD19+ B220+) during NP-LPS immunization. (E) Representative dot plots for PB gating in the spleen of both WT and Cxcr4+/1013 mice 6 days after NP-LPS immunization. (F) Percentage and total number of PBs in the spleen at days 0, 3 and 6 after immunization. (G) Quantification of total IgM+ and NP15-specific IgM+ ASCs in the spleen by ELISpot at days 0, 3, and 6 after immunization. (H) Representative staining of spleen sections from WT and Cxcr4+/1013 mice at day 3 after immunization. PBs are stained with an anti-IgM Ab (red), basal membrane is stained with an anti-laminin Ab (green), and nuclei are stained with Hoechst 33342 (Blue), Scale bar: 800 µm. (I) Quantification of IgM+ PB accumulation within the spleen at days 3 and 6. (J) Frequency of total PBs and of IgM+ ASCs determined by flow cytometry and ELISpot, respectively, in the spleen 6 days after NP-Ficoll immunization. (K) Serum titers of total IgM from both genotypes were measured by ELISA at days 0 and 6 after NP-Ficoll immunization. Results are from 3 independent experiments (A-G, J-K) or 1 representative experiment of 2 (H-I) (mean ± SEM, n = 2-7). Mann-Whitney U test was used to assess statistical significance except for panel I, where the Student t test was used (*P < .05, **P < .01, ***P < .001).
