Figure 1.
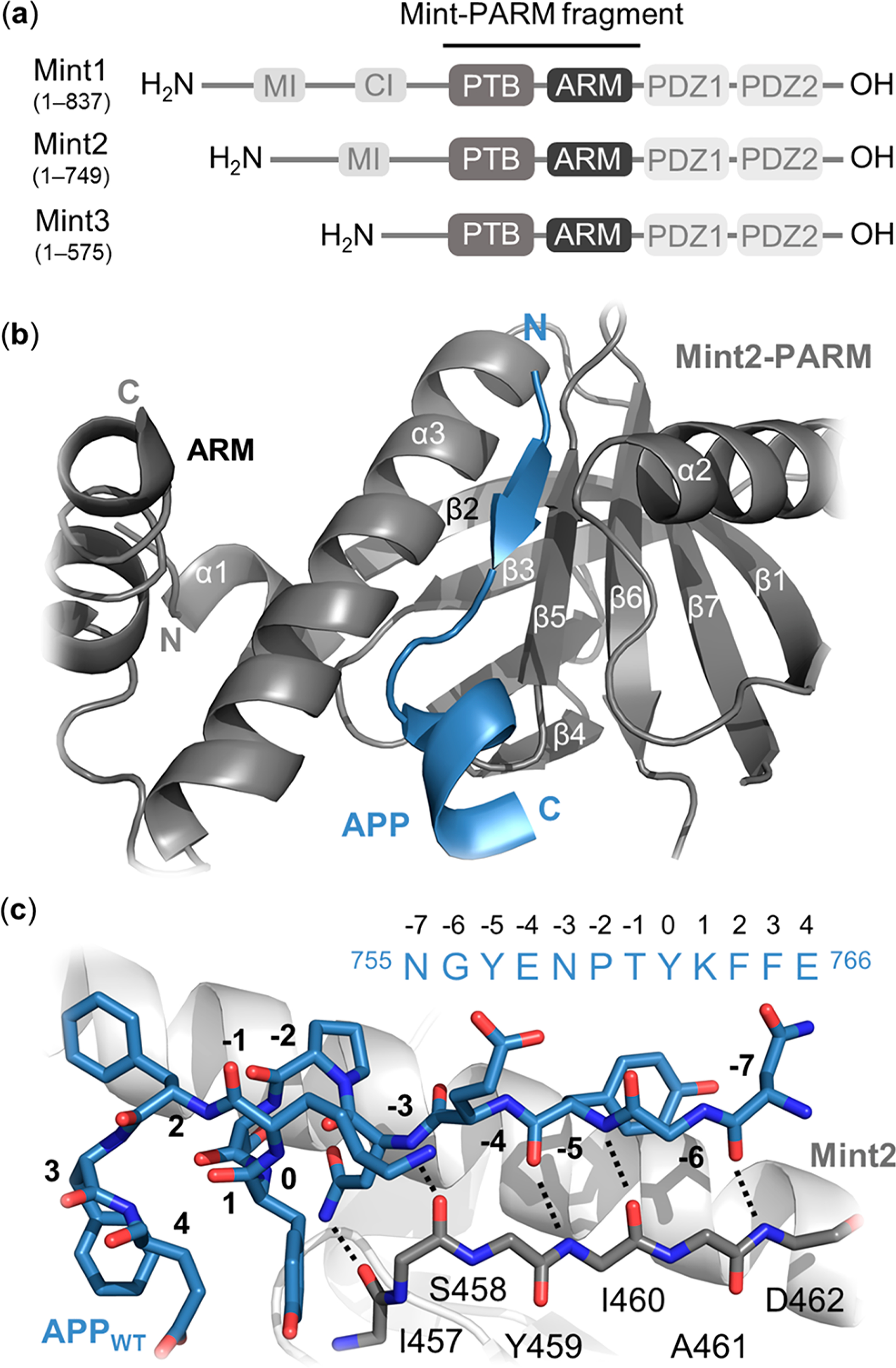
Structural overview of the Mint protein family and the Mint2-APP interaction. (a) Schematic architecture of Mint proteins illustrating their conserved C-terminal region consisting of a phosphotyrosine binding (PTB) domain (gray), an α-helical ARM linker (dark gray), two PSD-95/drosophila discs large/zonula occludens (ZO-1) (PDZ) domains (light gray), and a variable N-terminal region. Numbering corresponds to human residues. MI = Munc-18 interaction domain, CI = CASK interaction domain. (b) Cartoon representation of the interaction between rat Mint2-PARM (gray) and APP (residues 754–767; blue). The APPC-term is binding at the interface between the α3-helix and β5-strand of Mint2, forming an antiparallel β-sheet (N-terminal) followed by a β-turn and a C-terminal α-helix. The ARM linker (dark gray) is found in an open conformation enabling APP binding. (c) Structure of the APP peptide (blue sticks) bound to the rat Mint2-PTB domain (gray). The β5-strand of the PTB domain (gray stick, side chains not depicted) is shown to highlight the backbone–backbone H-bond network (black dotted lines) between the Mint2-PTB domain and APP (PDB ID: 3SV1).12
