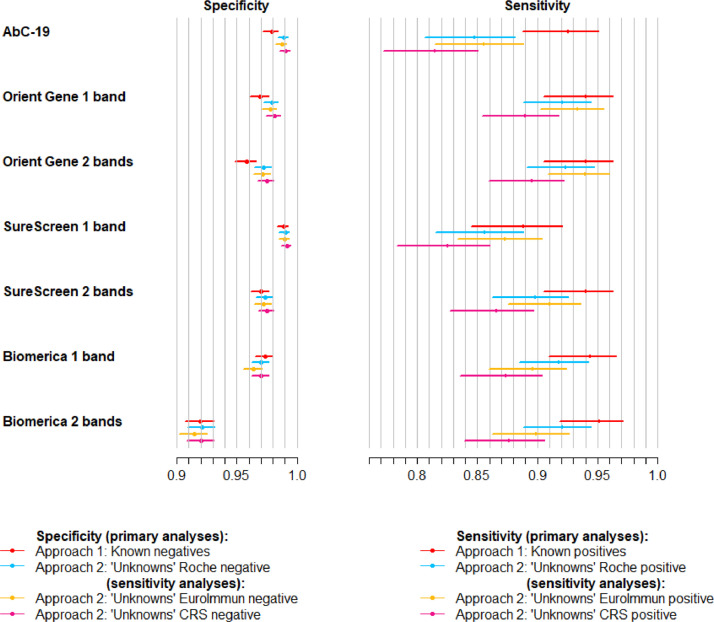
Fig. 2.
Sensitivity and specificity of lateral flow devices, with 95% confidence intervals. Four sets of estimates are shown: (i) Approach 1, i.e. specificity from analysis of known negatives and sensitivity from known positives (sample size: n = 1,995 for specificity, n = 268 for sensitivity); (ii) Approach 2 analysis of individuals with unknown previous infection status (“unknowns”), calculated against Roche Elecsys® reference standard (sample size: n = 2,225 for specificity, n = 354 for sensitivity); (iii) Approach 2 sensitivity analysis: analysis of unknowns compared with alternative EuroImmun reference standard (n = 2,233 for specificity, n = 346 for sensitivity); (iv) Approach 2 sensitivity analysis: analysis of unknowns compared with alternative composite reference standard (CRS) of positive on either Roche Elecsys® or EuroImmun versus negative on both (n = 2,207 for specificity, n = 372 for sensitivity.