Abstract
Background
The aim of this study was to characterize severe immune‐related adverse events (irAEs) seen among hospitalized patients and to examine risk factors for irAE admissions and clinically relevant outcomes, including length of stay, immune checkpoint inhibitor (ICI) discontinuation, readmission, and death.
Methods
Patients who received ICI therapy (ipilimumab, pembrolizumab, nivolumab, atezolizumab, durvalumab, avelumab, or any ICI combination) at Massachusetts General Hospital (MGH) and were hospitalized at MGH following ICI initiation between January 1, 2011, and October 24, 2018, were identified using pharmacy and hospital admission databases. Medical records of all irAE admissions were reviewed, and specialist review with defined criteria was performed. Demographic data, relevant clinical history (malignancy type and most recent ICI regimen), and key admission characteristics, including dates of admission and discharge, immunosuppressive management, ICI discontinuation, readmission, and death, were collected.
Results
In total, 450 admissions were classified as irAE admissions and represent the study's cohort. Alongside the increasing use of ICIs at our institution, the number of patients admitted to MGH for irAEs has gradually increased every year from 9 in 2011 to 92 in 2018. The hospitalization rate per ICI recipient has declined over that same time period (25.0% in 2011 to 8.5% in 2018). The most common toxicities leading to hospitalization in our cohort were gastrointestinal (30.7%; n = 138), pulmonary (15.8%; n = 71), hepatic (14.2%; n = 64), endocrine (12.2%; n = 55), neurologic (8.4%; n = 38), cardiac (6.7%; n = 30), and dermatologic (4.4%; n = 20). Multivariable logistic regression revealed statistically significant increases in irAE admission risk for CTLA‐4 monotherapy recipients (odds ratio [OR], 2.02; p < .001) and CTLA‐4 plus PD‐1 combination therapy recipients (OR, 1.88; p < .001), relative to PD‐1/PD‐L1 monotherapy recipients, and patients with multiple toxicity had a 5‐fold increase in inpatient mortality.
Conclusion
This study illustrates that cancer centers must be prepared to manage a wide variety of irAE types and that CTLA‐4 and combination ICI regimens are more likely to cause irAE admissions, and earlier. In addition, admissions for patients with multi‐organ involvement is common and those patients are at highest risk of inpatient mortality.
Implications for Practice
The number of patients admitted to Massachusetts General Hospital for immune‐related adverse events (irAEs) has gradually increased every year and the most common admissions are for gastrointestinal (30.7%), pulmonary (15/8%), and hepatic (14.2%) events. Readmission rates are high (29% at 30 days, 49% at 180 days) and 64.2% have to permanently discontinue immune checkpoint inhibitor therapy. Importantly, multiple concurrent toxicities were seen in 21.6% (97/450) of irAE admissions and these patients have a fivefold increased risk of inpatient death.
Keywords: Hospital admissions, Immune‐related adverse events
Short abstract
Real‐world immune‐related adverse events (irAE) studies have been hampered by the relatively recent introduction of immune checkpoint inhibitor therapy. This article characterizes severe irAEs among hospitalized patients and examines risk factors for irAE admissions and clinically relevant outcomes.
Introduction
Since ipilimumab was approved in 2011 for the treatment of advanced melanoma [1], the use of immune checkpoint inhibitors (ICIs) to treat cancer has rapidly grown. Currently, seven ICIs are approved, with new indications accumulating at a rapid pace. In 2011, only 1.5% of patients with cancer in the United States were eligible for ICIs; however, by 2019 the percentage had risen to 36.1% [2]. For several types of advanced cancer, most patients now receive an ICI as part of their first‐line therapy [3]. In parallel, ICI‐induced immune‐related adverse events (irAEs) are also increasing [4, 5], underscoring the importance of research into their incidence, type, severity, and mitigation.
Much of the current data on irAEs are derived from the clinical trial experience and, to date, real‐world irAE studies have been hampered by the relatively recent introduction of ICI therapy, which limits the sample sizes of irAE occurrences and hospitalizations [6]. Furthermore, the process of identifying and characterizing irAE hospital admissions, often through review of patient charts or databases, is complicated and without clear definitions [7]. Among clinical trials, reported rates of treatment‐related grade ≥ 3 irAEs have ranged from 0% to 42% [8, 9, 10, 11, 12, 13, 14, 15, 16], and serious irAE rates have ranged from 3% to 21% [8, 9, 14, 15, 16, 17]. In addition to varying ICI toxicity profiles (e.g., combination ICI regimen may carry increased risk of severe or life‐threatening irAEs compared with ICI monotherapy [18]), this discrepancy may be caused by the absence of accurate definitions and classification paradigms for irAEs, as there is not a gold standard grading criteria specific for toxicities secondary to ICIs.
The aim of this study was to characterize severe irAEs seen among hospitalized patients and to examine risk factors for irAE admissions and clinically relevant outcomes, including length of stay (LOS), ICI discontinuation, readmission, and death. It addresses prior sample size and disease criteria limitations by including all 450 irAE hospitalizations at a major academic medical system over 8 years and a multistep review process, including specialists with irAE expertise to confirm all irAEs along standardized diagnostic criteria.
Methods
This study was approved by the Partners Human Research Committee, the institutional review board of Partners HealthCare (#2017P000501).
Hospital Admission Review Process
Patients who received ICI therapy (ipilimumab, pembrolizumab, nivolumab, atezolizumab, durvalumab, avelumab, or any ICI combination) at Massachusetts General Hospital (MGH) and were hospitalized at MGH following ICI initiation between January 1, 2011, and October 24, 2018, were identified using pharmacy and hospital admission databases. Admissions underwent a multistage review process. Each was screened broadly for the presence of a potential irAE based on documentation in the electronic health record, including hospitalization records and primary inpatient team suspicion. These potential irAE admissions, categorized by type of toxicity, were then sent to the appropriate specialist with expertise in ICIs at our institution (allergy: JRF; cardiology: TGN, DAZ; dermatology: STC; endocrinology: ATF, MR; gastroenterology/hepatology: MLD, MFT; hematology: RSKL; nephrology: MES; neurology: ACG; pulmonology: DO, BDM; rheumatology: MN, MK, SS) for chart review and determination of irAE likelihood as “confirmed,” “suspected,” or “not toxicity.” Specialists followed consistent organ system–specific diagnostic criteria (supplemental online Table 1). Only “confirmed” and “suspected” cases were considered “irAE admission” for subsequent analyses.
Table 1.
Characteristics of ICI recipients and patients admitted for IrAE
| Characteristic | ICI recipients a | Patients admitted for IrAE b | IrAE admission rates c |
|---|---|---|---|
| Total ICI recipients | 2,884 | 344 (11.9) | |
| Age, mean (SD), years | 65.1 (13.2) | 64.5 (13.1) | |
| Female sex | 1,220 | 132 (38.3) | |
| Cancer type | |||
| Melanoma | 994 | 165 (48.0) | 16.6 |
| Thoracic | 776 | 77 (22.4) | 9.9 |
| Gastrointestinal | 354 | 43 (12.5) | 12.2 |
| Head and neck | 226 | 7 (2.0) | 3.1 |
| Genitourinary | 164 | 17 (4.9) | 10.4 |
| Gynecologic | 122 | 9 (2.6) | 7.4 |
| Hematologic | 89 | 10 (2.9) | 11.2 |
| Neurologic | 82 | 8 (2.3) | 9.8 |
| Breast | 60 | 7 (2.0) | 11.7 |
| Sarcoma | 17 | 1 (0.93) | 5.9 |
| ICI type | |||
| PD‐1/PD‐L1 | 2,265 | 223 (67.7) | 9.9 |
| CTLA4 | 355 | 67 (19.5) | 18.9 |
| CTLA4 + PD1 | 264 | 54 (15.7) | 20.5 |
Total number of ICI recipients for corresponding characteristic, unless otherwise indicated.
Data are presented as number (percentage) of patients admitted for irAE (excluding readmissions) unless otherwise indicated.
Data are presented as percentage of patients admitted for irAE within each row category (cancer and ICI type).
Abbreviations: CI, confidence interval; CTLA‐4, cytotoxic T‐lymphocyte antigen‐4; ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event; OR, odds ratio; PD‐1, programmed death‐1; PD‐L1, programmed death‐ligand 1; SD, standard deviation.
Data Collection
Medical records of all irAE admissions were reviewed. Demographic data, relevant clinical history (malignancy type and most recent ICI regimen), and key admission characteristics, including dates of admission and discharge, immunosuppressive management, ICI discontinuation, readmission, and death during irAE hospitalization, were collected. In cases of multiple confirmed toxicities, the primary irAE was defined as that which prompted hospitalization and/or determined treatment.
Statistical Analysis
Statistical analyses were performed using Stata, version 15.0 (StataCorp, College Station, TX). Multivariate logistic and linear regressions were used to predict risk of irAE admission, time to admission, length of stay, treatment with corticosteroids and nonsteroidal immunosuppression, ICI discontinuation, irAE readmission, and inpatient mortality, adjusted for age, sex, admission year, malignancy, ICI class, irAE confirmation status, primary irAE, and presence of multiple toxicities. Multinomial logistic regression was used to determine baseline characteristics associated with the development of each primary irAE. A p value of <.05 was considered statistically significant. Benjamini‐Hochberg procedure was performed for a false discovery rate of 5%, and only those p values that remained significant after this correction are presented.
Results
After reviewing 2,726 hospitalizations following ICI initiation in 2,884 ICI patients between January 1,2011, and October 24, 2018, 688 (25.2%) screened positive for potential irAE (Fig. 1). Following expert specialist review, 238 (34.6% of 688 positive screens) were removed. In total, 450 admissions were classified as irAE admissions and represent our study's cohort (258 “confirmed” and 192 “suspected,” as per expert classification).
Figure 1.
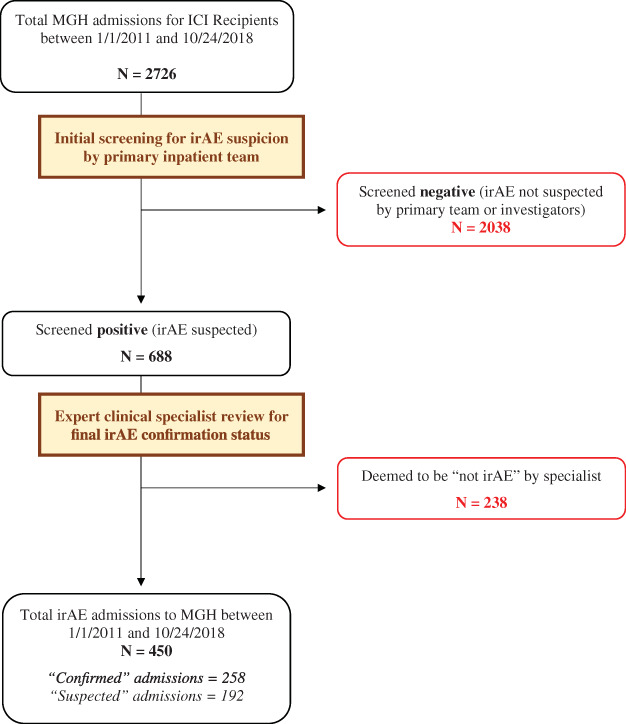
Study flow diagram. Flow diagram of study's methodology, hospitalization screening, and final case selection.
Abbreviations: ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event; MGH, Massachusetts General Hospital.
Temporal Trends: 2011–2018
Alongside the increasing use of ICIs at our institution (Fig. 2A), the number of patients admitted to MGH for irAEs has gradually increased every year from 9 in 2011 to 92 in 2018 (Fig. 2B). The hospitalization rate per ICI recipient has declined over that same time period (25.0% in 2011 to 8.5% in 2018), though it has remained steady since 2015. Nevertheless, the proportion of total MGH Cancer Center admissions that are due to irAEs has grown from 0.40% in 2011 to 2.86% in 2017 (R2 = 0.752; Fig. 2C).
Figure 2.
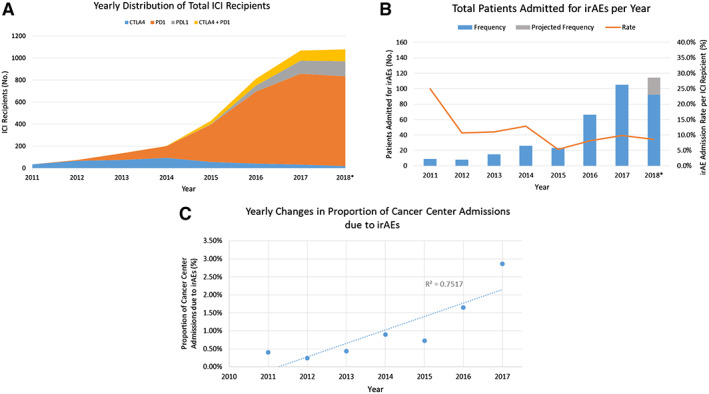
General yearly trends in ICI recipients and irAE admissions. (A): Increasing annual use of ICIs at MGH, driven largely by PD‐1 therapy (orange) in recent years. (B): Absolute number of patients admitted for irAEs has increased annually (grey bar represents projected 2018 full‐year data based on our 10‐month data), but irAE patient admission rate per ICI recipient has declined annually (orange line). (C): IrAE admissions account for a growing share of total Cancer Center admissions at our institution (dotted best‐fit line; R2 = 0.752).*, data end October 24, 2018.
Abbreviations: ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event; MGH, Massachusetts General Hospital.
Although patients with melanoma accounted for >95% of all irAE admissions from 2011 to 2015, their fraction of admissions dropped to 26.5% by 2018. Patients with thoracic and gastrointestinal (GI) cancers now make up a substantial proportion of admitted patients (Fig. 3A). Similarly, ICI regimens of hospitalized patients have also changed over time. The leading ICI class administered prior to irAE admissions has transitioned from CTLA‐4 monotherapy before 2015 (73.2%; 30/41) to PD‐1 monotherapy after 2015 (71.7%; 81/113; Fig. 3B).
Figure 3.

Distribution trends in cancer, ICI, and toxicity types among irAE admissions. Yearly irAE admission trends by cancer and ICI type reflect changing landscape of ICI use with increasing diversity of malignancies (A) and increasing PD‐1 representation (B) after 2015. Although gastrointestinal toxicities (light blue) drove the majority of all irAE admissions from 2011 through 2015, there has been a consistently diverse mix of inpatient irAEs after 2015 (C). *, data end October 24, 2018.
Abbreviations: CTLA‐4, cytotoxic T‐lymphocyte antigen‐4; ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event; MGH, Massachusetts General Hospital.
The most common toxicities leading to hospitalization in our cohort were GI (30.7%; 138/450), pulmonary (15.8%; 71/450), hepatic (14.2%; 64/450), endocrine (12.2%; 55/450), neurologic (8.4%; 38/450), cardiac (6.7%; 30/450), and dermatologic (4.4%; 20/450). No other toxicity type exceeded 3% of admissions. From 2011 through 2015, GI toxicities represented the majority of all irAE admissions. However, after 2015, the spectrum of primary inpatient irAEs rapidly diversified, with each of the 11 different toxicity types accounting for 20% or less of all admissions (Fig. 3C).
Risk Factors and Timing of irAE Admissions
Among all 2,884 ICI recipients at our institution, 344 patients (mean age, 64.5 +/− 13.1 years; 38.4% women) were admitted for irAE (450 total admissions and readmissions occurring in 344 patients), representing an admission rate of 11.93% across an 8‐year period (Table 1). IrAE admission rates by malignancy type ranged from the lowest at 3.1% for head and neck cancers to the highest at 16.6% for melanoma. Multivariable logistic regression revealed statistically significant increases in irAE admission risk for CTLA‐4 monotherapy recipients (odds ratio [OR], 2.02; 95% confidence interval [CI], 1.37–2.99; p < .001) and CTLA‐4 plus PD‐1 combination therapy recipients (OR, 1.88; 95% CI, 1.33–2.67; p < .001), relative to PD‐1/PD‐L1 monotherapy recipients. Age and sex were not significant predictors of irAE admission. On multinomial logistic regression, adjusting for age, sex, malignancy, and ICI type, patients with thoracic cancer had a significantly increased risk of admission for pulmonary toxicity (relative risk ratio, 6.81; 95% CI, 2.44–19.02; p < .001) relative to patients with melanoma. Of note, 14/344 patients had underlying autoimmune conditions at baseline. A total of 5/14 of these patients were on disease‐modifying therapy at baseline (supplemental online Table 2), which included (a) prednisone 5 mg + hydroxychloroquine for polymyalgia rheumatic, (b) prednisone 5 mg for rheumatoid arthritis, (c) leflunomide for rheumatoid arthritis, (d) low‐dose azathioprine for ulcerative colitis, and (e) sulfasalazine for ulcerative colitis.
Table 2.
Factors associated with ICI discontinuation, irAE readmission, and inpatient mortality
| Outcome (total n) | n (%) | OR (95% CI) | p value a |
|---|---|---|---|
| ICI discontinuation (279) b | 179 (64.1) | ||
| Cardiac irAE (18) | 16 (88.9) | 6.30 (1.14–34.71) c | .034 |
| Dermatologic irAE (15) | 3 (20.0) | 0.18 (0.04–0.77) c | .021 |
| Hepatic irAE (45) | 32 (71.1) | 2.82 (1.12–7.13) c | .028 |
| IrAE readmission (425) d | 105 (24.7) | ||
| PD‐1/PD‐L1 (259) | 52 (20.0) | – base variable – | |
| CTLA4 (93) | 27 (29.0) | 1.01 (0.37–2.70) e | .989 |
| CTLA4 + PD1 (73) | 26 (35.6) | 3.93 (1.65–9.37) e | .002 |
| ICI discontinuation for irAE (190) b | 29 (15.3) | 0.16 (0.08–0.31) e | <.001 |
| Inpatient mortality (450) f | 25 (5.6) | ||
| Multiple irAEs (97) | 8 (8.3) | 5.27 (1.63–17.03) c | .005 |
Significant p values are in bold.
ICI discontinuation excludes all endocrine toxicities (for which ICI discontinuation is not recommended), patients who had previously discontinued ICI prior to admission, patients whose ICI was discontinued for nontoxicity reasons (i.e., disease progression), and patients who died during hospitalization.
Multivariable logistic regression with covariates: age, sex, year, irAE confirmation status, malignancy (base melanoma), and ICI class (base PD‐1/PD‐L1), primary toxicity type (base gastrointestinal), and presence of multiple toxicities.
Readmission for any toxicity following previous irAE admission from the same culprit ICI regimen. Excludes admissions that resulted in death.
Multivariable logistic regression with covariates: age, sex, year, irAE confirmation status, malignancy (base melanoma), and ICI class (base PD‐1/PD‐L1), primary toxicity type (base gastrointestinal), presence of multiple toxicities, and ICI discontinuation for toxicity.
Inpatient mortality only counts patients who died during their hospitalization for irAE.
Abbreviations: CI, confidence interval; CTLA‐4, cytotoxic T‐lymphocyte antigen‐4; ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event; SD, standard deviation; OR, odds ratio; PD‐1, programmed death‐1; PD‐L1, programmed death‐ligand 1.
Median time from ICI initiation to first irAE admission was 61 days (interquartile range [IQR], 28–128.5 days). Time to admission varied significantly by ICI type and by type of irAE (Fig. 4A and 4B). In multivariable regression modeling, treatment with CTLA‐4 monotherapy (coefficient, −81.1; 95% CI, −148.6 to −13.6; p = .019) and CTLA‐4 plus PD‐1 combination therapy (coefficient, −102.5; 95% CI, −158.6 to −46.5; p < .001) were associated with significantly earlier time to irAE admission relative to treatment with PD‐1/PD‐L1 monotherapy.
Figure 4.
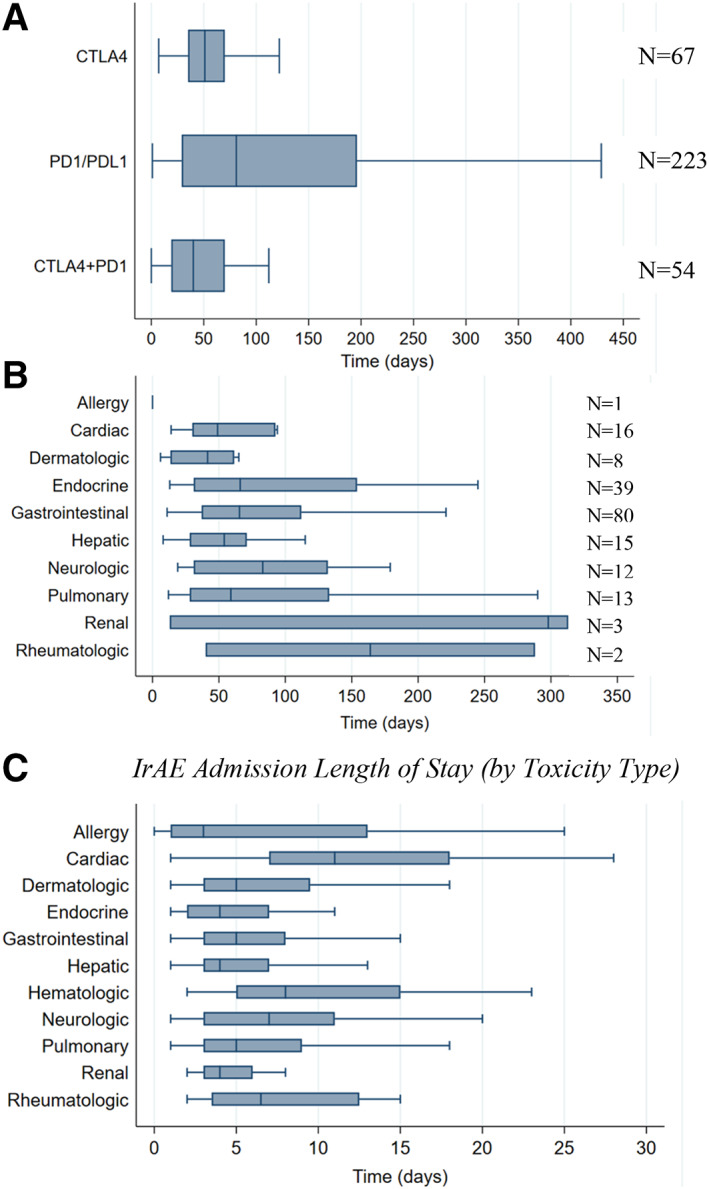
Time to first admission and length of stay of irAE admissions. (A): Time to first irAE admission was lower among CTLA‐4 monotherapy (median, 51; IQR, 35–70; p = .019) and CTLA‐4 plus PD‐1 combination therapy recipients (median, 40; IQR, 19–70; P < .001) compared with PD‐1/PD‐L1 monotherapy recipients (median, 81; IQR, 29–196). (B): Although not statistically significantly different, time to admission varied by type of confirmed irAE. (C): Length of stay per irAE admission varied significantly by toxicity type, with cardiac (median, 11; IQR, 7–18; p < .001) and neurologic irAEs (median, 7; IQR, 3–11; p = .027) associated with statistically significant increases in length of stay on multivariable linear regression. Box plots do not display outlier observations.
Abbreviations: CTLA‐4, cytotoxic T‐lymphocyte antigen‐4; ICI, immune checkpoint inhibitor; IQR, interquartile range; irAE, immune‐related adverse event.A. Time from ICI initiation to first irAE admission (by ICI and confirmed toxicity type) B. IrAE admission length of stay (by toxicity type)
Multiple Inpatient Toxicities
Multiple concurrent toxicities were seen in 21.6% (97/450) of irAE admissions. Among ICI regimens, patients on CTLA‐4 plus PD‐1 combination therapy had the highest rate of multiple toxicities at 35.9% (28/78) of admissions, followed by CTLA‐4 monotherapy at 22.6% (21/93) and PD‐1/PD‐L1 monotherapy at 17.2% (48/279). On multivariable logistic regression, age (OR, 1.03; 95% CI, 1.01–1.05; p = .016), female sex (OR, 2.67; 95% CI, 1.56–4.56; p < .001), and combination therapy (OR, 3.75; 95% CI, 1.79–7.85; p < .001) were all associated with significantly increased risk of multiple toxicities during irAE admission.
Patient‐Related Outcomes: Length of Stay, Inpatient Management, ICI Discontinuation, Readmission, and Mortality
Median LOS among our 450 irAE admissions was 5 days (IQR, 3–9 days). Lengths of stay were similar across all cancer types and culprit ICI regimens. Among toxicity types (Fig. 4C), admission for cardiac irAE (median, 11; IQR, 7–18) was associated with a statistically significant increase in LOS in multivariable regression modeling (coefficient, 5.70; 95% CI, 2.63–8.76; p < .001) relative to admission for GI toxicity.
Excluding patients who died during hospitalization and patients with thyroid toxicities and diabetes mellitus irAE, for which steroid treatment is not indicated, patients were discharged on systemic corticosteroids for their presenting toxicities in 80% (324/405) of irAE admissions. Patients with GI toxicity had the highest rate of steroid usage on discharge (91.9%; 125/136). Patients received second‐line, nonsteroidal immunosuppressive treatments in 14.5% (65/450) of irAE admissions. Second‐line immunosuppression was relatively common for inpatient hematologic (30%; 3/10), GI (25.4%; 35/138), and cardiac (23.3%; 7/30) toxicities.
Excluding all endocrine toxicities (for which ICI discontinuation is not recommended), patients who discontinued ICI treatment prior to admission or for non‐irAE reasons, and patients who died during hospitalization, 64.2% (179/279) of irAE admissions resulted in permanent ICI discontinuation because of presenting toxicity (Table 2). In multivariable regression modeling, significantly increased risk of ICI discontinuation was seen among admissions for cardiac (89%; OR, 6.30; p = .034) and hepatic irAEs (71%; OR, 2.82; p = .028). Inpatient dermatologic irAEs were associated with significantly decreased risk of ICI discontinuation (20%; OR, 0.18; p = .021).
Among the 324 first‐time admitted patients who did not die during hospitalization, 73 (22.5%) were readmitted at least once for any irAE. Within 30 days of first irAE admission, 17.6% (57/324) of patients were readmitted for any irAE and 29.3% (95/324) were readmitted for any reason. Within 180 days of first irAE admissions, 21.6% (70/324) of patients were readmitted for any irAE and 49.1% (159/324) were readmitted for any reason. IrAE readmission was most common among GI (30.9%; 42/136), neurologic (29.7%; 11/37), and cardiac irAEs (29.6%; 8/27). Patients who discontinued ICI therapy for inpatient irAE were less likely to be readmitted for any irAE compared with those who continued therapy (15.3% vs. 43.3%; OR, 0.16; 95% CI, 0.08–0.31; p < .001). In multivariable regression modeling, combination treatment with CTLA‐4 and PD‐1 significantly increased the risk of readmission for irAE (OR, 3.93 95% CI, 1.65–9.37; p = .002; Table 2).
The overall inpatient mortality rate due to irAE in our study was 5.6% (25/450). Although age, sex, and type of cancer, ICI, or toxicity had no statistically significant impact on mortality, inpatient mortality rates were numerically highest for pulmonary (15.5%; 11/71) and cardiac irAEs (10.0%; 3/30). In multivariable regression modeling, only the presence of multiple concurrent toxicities was associated with a statistically significant increase in inpatient mortality risk (8.3%; OR, 5.27; 95% CI, 1.63–17.03; p = .005; Table 2).
Discussion
This study characterizes our institution's inpatient experience with irAEs, including predictors of toxicity and clinical outcomes. In doing so, we revealed multiple findings that hospitals may use to optimize care of these complex patients.
The admission rate for patients receiving ICIs at our institution declined over time from 25.0% in 2011 to 8.5% in 2018. This change, as well as the fairly steady irAE hospitalization rate since 2015, may reflect more favorable toxicity profiles of newer ICI regimens, improved confidence in outpatient management of irAEs, or increasingly high thresholds for hospitalization. However, even as the irAE admission rate declined, irAE hospitalizations began to account for a growing share of total oncology admissions, increasing from 0.4% in 2011 to 2.9% in 2017. The distribution of toxicity types also changed dramatically over time: whereas gastrointestinal irAEs drove the bulk of admissions in the early years of ICI use, after 2015 they dropped to levels equivalent to those for pulmonary, hepatic, neurologic, and endocrine toxicities. This change may reflect the increasing diversity of ICI regimens and oncologic indications for ICIs, both of which may influence the range of toxicities to which patients are susceptible. In practice, this means that cancer centers must be ready to detect and manage a much greater diversity of irAEs in consultation with specialist teams.
In investigating risk factors for irAE hospitalization, we found that CTLA‐4 therapy or combination ICI therapy was associated with a nearly twofold increase in irAE admission risk relative to PD‐1/PD‐L1 therapy. This is consistent with previous findings that combination ICI therapy is a risk factor for the occurrence of severe or life‐threatening irAEs, often requiring hospitalization [18, 19]. Furthermore, patients with severe toxicities caused by CTLA‐4 therapy and CTLA‐4 plus PD‐1 combination therapy were admitted significantly earlier in treatment (at 6 to 7 weeks) than those caused by PD‐1/PD‐L1 monotherapy (at 3 months). Taken together, our findings suggest that patients on these higher‐risk therapies should be monitored closely for the earliest signs of emerging toxicity. Of note, we did not find an association between patient age and risk of irAE hospitalization. This is consistent with the results of one previous real‐world study [20], though not with findings from other studies, in which either younger [21] or older [19] age was significantly associated with irAE admission risk.
The overall irAE admission rate of 11.9% per ICI recipient in this study was markedly lower than the 41% rate for suspected irAEs and the 23% rate for confirmed irAEs recently reported in another study at a major academic center [19]. This wide variation may reflect differences in the ways irAEs were defined, categorized, and confirmed or variability in outpatient processes of care.
Notably, multiple concurrent toxicities were present in over 20% of irAE admissions. Multiple irAEs have been infrequently described in the literature before; for example, one study reported that 26.1% of patients hospitalized for irAEs experienced multiple toxicities [19]. However, in our study, we were able to investigate factors predictive of multiple irAEs. Older age, women, and patients treated with combination ICI therapy were all significantly more likely to be admitted with multiple irAEs. Importantly, patients with multiple concurrent toxicities had greater than five times the risk of inpatient death than patients with single toxicities.
In our study, median length of stay for irAE admissions was 5 days. This is slightly shorter than the median 6‐day length of stay previously reported for irAE admissions [19]. However, admissions for cardiac irAEs had a median 11‐day length of stay, suggesting that this toxicity may require more extensive diagnostic workup or therapeutic monitoring during hospitalization [22, 23, 24, 25].
Among eligible admissions (i.e., excluding all endocrine toxicities, patients who discontinued therapy prior to admission or for nontoxicity reasons, and patients who died during hospitalization), 64% of irAE admissions resulted in ICI discontinuation. This rate is lower than previously reported (87%) [19]. This difference may reflect a discrepancy in the way that the institutions manage irAEs or in study methodology. At our institution, patients with cardiac and hepatic irAE admissions were at highest risk of ICI discontinuation, whereas patients with dermatologic irAEs were at significantly lower risk. Although permanent ICI discontinuation reflects a significant disruption to a patient's oncologic management plan, further studies are needed to elucidate the impact of early ICI termination on long‐term survival outcomes [26, 27, 28].
Analyzing risk factors for readmission, we found that ICI discontinuation strongly protects against rehospitalization for any subsequent irAE, highlighting the impact that prompt ICI discontinuation can have on the course of severe toxicities. A quarter of all irAE admissions in our study were readmissions, and we had a 29% all‐cause 30‐day readmission rate, a figure that underscores the importance of providing close outpatient monitoring for patients once they are discharged.
Finally, 5.6% of irAE admissions in this study ended in death. This number likely underestimates the actual mortality rate associated with irAE hospitalization, as it does not capture patients discharged to hospice who died soon thereafter. It is similar to the 6.0% mortality rate reported in a study of patients who experienced clinically significant irAEs at another major hospital [29]. The only predictor of inpatient mortality that we identified was the presence of multiple, concurrent irAEs during admission, emphasizing the need for multidisciplinary collaboration for such high‐risk, complex patients. Patient age, over and above its effect on multiple toxicities, was not independently predictive of mortality, though a previous study found that older patients were more likely to die when hospitalized for an irAE [21].
Our study is limited primarily by its single‐institution and retrospective design that may have missed patients who were admitted to MGH for irAE but had received ICI treatment elsewhere as well as MGH's ICI recipients who were admitted elsewhere. Nevertheless, our study also has several key strengths. It characterizes the largest group of irAE admissions from a single institution analyzed to date, over a time period sufficiently long to elucidate temporal trends in hospitalizations. In addition, it employed a multistep review process in which every irAE was confirmed by a relevant specialist by strict and consistent diagnostic criteria. Specialist review resulted in rejection of over one third of irAEs suspected by the primary inpatient team, demonstrating the importance of including this step in irAE studies to exclude hospitalizations not from irAEs. This high rate of irAE rejection by retrospective specialist review not only underscores the important contribution of specialist involvement in potentially ruling out irAEs when they are initially suspected among hospitalized patients but also may reflect the growing knowledge that nearly a decade of experience has afforded our specialist reviewers to confidently retrospectively remove cases that may have previously been suspicious for irAE when adhering to strict irAE admission definitions (supplemental online Table 1).
Conclusion
This study illustrates that cancer centers must be prepared to manage a wide variety of irAE types and that CTLA‐4 and combination ICI regimens are more likely to cause irAE admissions that occur earlier after drug initiation. Furthermore, we found that more than 20% of patients admitted for an irAE will experience multiple concurrent toxicities, which then carries a fivefold increased risk of inpatient death, identifying this population as a very high‐risk group. These results emphasize the importance of having a robust multidisciplinary care team in place to promptly identify irAEs and provide safe and effective management of medically complex patients with multisystem toxicities. Going forward, it will be critical to investigate the effectiveness of different institutional strategies for managing inpatient irAEs to identify those that result in the best outcomes for patients.
Author Contributions
Conception/design: Gabriel E. Molina, Leyre Zubiri, Justine V. Cohen, Sienna M. Durbin, Laura Petrillo, Ian M. Allen, Yonina R. Murciano‐Goroff, Michael L. Dougan, Molly F. Thomas, Alexander T. Faje, Michelle Rengarajan, Amanda C. Guidon, Steven T. Chen, Daniel Okin, Benjamin D. Medoff, Mazen Nasrallah, Minna Kohler, Sara Schoenfeld, Rebecca S. Karp Leaf, Meghan E. Sise, Tomas G. Neilan, Daniel A. Zlotoff, Jocelyn R. Farmer, Meghan Mooradian, Aditya Bardia, Minh Mai, Ryan J. Sullivan, Yevgeniy R. Semenov, Alexandra C Villani, Kerry L. Reynolds
Provision of study material or patients: Gabriel E. Molina, Leyre Zubiri, Justine V. Cohen, Sienna M. Durbin, Laura Petrillo, Ian M. Allen, Yonina R. Murciano‐Goroff, Michael L. Dougan, Molly F. Thomas, Alexander T. Faje, Michelle Rengarajan, Amanda C. Guidon, Steven T. Chen, Daniel Okin, Benjamin D. Medoff, Mazen Nasrallah, Minna Kohler, Sara Schoenfeld, Rebecca S. Karp Leaf, Meghan E. Sise, Tomas G. Neilan, Daniel A. Zlotoff, Jocelyn R. Farmer, Meghan Mooradian, Aditya Bardia, Minh Mai, Ryan J. Sullivan, Yevgeniy R. Semenov, Alexandra C Villani, Kerry L. Reynolds
Collection and/or assembly of data: Gabriel E. Molina, Leyre Zubiri, Justine V. Cohen, Sienna M. Durbin, Laura Petrillo, Ian M. Allen, Yonina R. Murciano‐Goroff, Michael L. Dougan, Molly F. Thomas, Alexander T. Faje, Michelle Rengarajan, Amanda C. Guidon, Steven T. Chen, Daniel Okin, Benjamin D. Medoff, Mazen Nasrallah, Minna Kohler, Sara Schoenfeld, Rebecca S. Karp Leaf, Meghan E. Sise, Tomas G. Neilan, Daniel A. Zlotoff, Jocelyn R. Farmer, Meghan Mooradian, Aditya Bardia, Minh Mai, Ryan J. Sullivan, Yevgeniy R. Semenov, Alexandra C Villani, Kerry L. Reynolds
Data analysis and interpretation: Gabriel E. Molina, Leyre Zubiri, Justine V. Cohen, Sienna M. Durbin, Laura Petrillo, Ian M. Allen, Yonina R. Murciano‐Goroff, Michael L. Dougan, Molly F. Thomas, Alexander T. Faje, Michelle Rengarajan, Amanda C. Guidon, Steven T. Chen, Daniel Okin, Benjamin D. Medoff, Mazen Nasrallah, Minna Kohler, Sara Schoenfeld, Rebecca S. Karp Leaf, Meghan E. Sise, Tomas G. Neilan, Daniel A. Zlotoff, Jocelyn R. Farmer, Meghan Mooradian, Aditya Bardia, Minh Mai, Ryan J. Sullivan, Yevgeniy R. Semenov, Alexandra C Villani, Kerry L. Reynolds
Manuscript writing: Gabriel E. Molina, Leyre Zubiri, Justine V. Cohen, Sienna M. Durbin, Laura Petrillo, Ian M. Allen, Yonina R. Murciano‐Goroff, Michael L. Dougan, Molly F. Thomas, Alexander T. Faje, Michelle Rengarajan, Amanda C. Guidon, Steven T. Chen, Daniel Okin, Benjamin D. Medoff, Mazen Nasrallah, Minna Kohler, Sara Schoenfeld, Rebecca S. Karp Leaf, Meghan E. Sise, Tomas G. Neilan, Daniel A. Zlotoff, Jocelyn R. Farmer, Meghan Mooradian, Aditya Bardia, Minh Mai, Ryan J. Sullivan, Yevgeniy R. Semenov, Alexandra C Villani, Kerry L. Reynolds
Final approval of manuscript: Gabriel E. Molina, Leyre Zubiri, Justine V. Cohen, Sienna M. Durbin, Laura Petrillo, Ian M. Allen, Yonina R. Murciano‐Goroff, Michael L. Dougan, Molly F. Thomas, Alexander T. Faje, Michelle Rengarajan, Amanda C. Guidon, Steven T. Chen, Daniel Okin, Benjamin D. Medoff, Mazen Nasrallah, Minna Kohler, Sara Schoenfeld, Rebecca S. Karp Leaf, Meghan E. Sise, Tomas G. Neilan, Daniel A. Zlotoff, Jocelyn R. Farmer, Meghan Mooradian, Aditya Bardia, Minh Mai, Ryan J. Sullivan, Yevgeniy R. Semenov, Alexandra C Villani, Kerry L. Reynolds
Disclosures
Yonina R. Murciano‐Goroff: AstraZeneca (H); Michael L. Dougan: Novartis (RF), Genentech (C/A); Amanda C. Guidon: Alexion, RaPharma (SAB), Momenta (C/A); Benjamin D. Medoff: Sanofi, Regeneron (SAB); Boehringer‐Ingelheim, Bayer, Celgene (RF); Tomas G. Neilan: Parexel, Bristol‐Myers Squibb, H3 Biomedicine, Aprea Therapeutics, Intrinsic Imaging (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1 Diagnostic Criteria for Subspecialist Review of irAE Admissions
TABLE S2. Patients with autoimmune conditions at baseline.
Disclosures of potential conflicts of interest may be found at the end of this article
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com.
References
- 1. Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for inhibitor checkpoint inhibitor drugs. JAMA Netw Open 2020;3:e200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandhi L, Rodríquez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 4. Wang DY, Salem J‐E, Cohen JV et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta‐analysis. JAMA Oncol 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moslehi JJ, Salem J‐E, Sosman JA et al. Increased reporting of fatal immune checkpoint inhibitor‐associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasello G, Pavan A, Attili I et al. Real world data in the era of immune checkpoint inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat Rev 2020;87:102031. [DOI] [PubMed] [Google Scholar]
- 7. Hsiehchen D, Watters MK, Lu R et al. Variation in the assessment of immune‐related adverse event occurrence, grade, and timing in patients receiving immune checkpoint inhibitors. JAMA Netw Open 2019;2:e1911519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gangadhar TC, Hwu WJ, Postow MA et al. Efficacy and safety of pembrolizumab in patients enrolled in KEYNOTE‐030 in the United States: An expanded access program. J Immunother 2017;40:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garassino MC, Cho BC, Kim JH et al. Durvalumab as third‐line or later treatment for advanced non‐small‐cell lung cancer (ATLANTIC): An open‐label, single‐arm, phase 2 study. Lancet Oncol 2018;19:521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antonia SJ, López‐Martin JA, Bendell J et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): A multicentre, open‐label, phase 1/2 trial. Lancet Oncol 2016;17:883–895. [DOI] [PubMed] [Google Scholar]
- 11. Migden MR, Rischin D, Schmults CD et al. PD‐1 blockade with cemiplimab in advanced cutaneous squamous‐cell carcinoma. N Engl J Med 2018;379:341–351. [DOI] [PubMed] [Google Scholar]
- 12. Sharma P, Retz M, Siefker‐Radtke A et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single‐arm, phase 2 trial. Lancet Oncol 2017;18:312–322. [DOI] [PubMed] [Google Scholar]
- 13. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balar AV, Castellano D, O'Donnell PH et al. First‐line pembrolizumab in cisplatin‐ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE‐052): A multicentre, single‐arm, phase 2 study. Lancet Oncol 2017;18:1483–1492. [DOI] [PubMed] [Google Scholar]
- 15. Overman MJ, McDermott R, Leach JL et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): An open‐label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 17. Zhu AX, Finn RS, Edeline J et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE‐224): A non‐randomised, open‐label phase 2 trial. Lancet Oncol 2018;19:940–952. [DOI] [PubMed] [Google Scholar]
- 18. Xu C, Chen YP, Du XJ et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta‐analysis. BMJ 2018;363:k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balaji A, Zhang J, Wills B et al. Immune‐related adverse events requiring hospitalization: Spectrum of toxicity, treatment, and outcomes. J Oncol Pract 2019;15:e825–e834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ksienski D, Wai ES, Croteau NS et al. Association of age with differences in immune related adverse events and survival of patients with advanced nonsmall cell lung cancer receiving pembrolizumab or nivolumab. J Geriatr Oncol 2020;11:807–813. [DOI] [PubMed] [Google Scholar]
- 21. Shah KP, Song H, Ye F et al. Demographic factors associated with toxicity in patients treated with anti‐programmed cell death‐1 therapy. Cancer Immunol Res 2020;8:851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahmood SS, Fradley MG, Cohen JV et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alvi RM, Frigault MJ, Fradley MG et al. Cardiovascular events among adults treated with chimeric antigen receptor T‐cells (CAR‐T). J Am Coll Cardiol 2019;74:3099–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Awadalla M, Mahmood SS, Groarke JD et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor‐related myocarditis. J Am Coll Cardiol 2020;75:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang L, Zlotoff DA, Awadalla M et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor–associated myocarditis. Circulation 2020;141:2031–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolchok JD, Chiarion‐Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schadendorf D, Wolchok JD, Hodi FS et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J Clin Oncol 2017;35:3807–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warner AB, Palmer JS, Shoushtari AN. Long‐term outcomes and responses to retreatment in patients with melanoma treated with PD‐1 blockade. J Clin Oncol 2020;38:1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eun Y, Kim IY, Sun JM et al. Risk factors for immune‐related adverse events associated with anti‐PD‐1 pembrolizumab. Sci Rep 2019;9:14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1 Diagnostic Criteria for Subspecialist Review of irAE Admissions
TABLE S2. Patients with autoimmune conditions at baseline.


