Abstract
Objectives
To develop a scoring tool, Pelvic Lymphadenectomy Appropriateness and Completion Evaluation (PLACE), to assess the intraoperative completeness and appropriateness of pelvic lymph node dissection (PLND) following robot-assisted radical cystectomy (RARC).
Patients, Subjects and Methods
A panel of 11 open and robotic surgeons developed the content and structure of PLACE. The PLND template was divided into three zones. In all, 21 de-identified videos of bilateral robot-assisted PLNDs were assessed by the 11 experts using PLACE to determine inter-rater reliability. Lymph node (LN) clearance was defined as the proportion of cleared LNs from all PLACE zones. We investigated the correlation between LN clearance and LN count. Then, we compared the LN count of 18 prospective PLNDs using PLACE with our retrospective series performed using the extended template (No PLACE).
Results
A significant reliability was achieved for all PLACE zones among the 11 raters for the 21 bilateral PLND videos. The median (interquartile range) for LN clearance was 468 (431–545). There was a significant positive correlation between LN clearance and LN count (R2 = 0.70, P < 0.01). The PLACE group yielded similar LN counts when compared to the No PLACE group.
Conclusions
Pelvic Lymphadenectomy Appropriateness and Completion Evaluation is a structured intraoperative scoring system that can be used intraoperatively to measure and quantify PLND for quality control and to facilitate training during RARC.
Keywords: robot-assisted, radical cystectomy, lymph node dissection, qualit, lymphadenectomy, #BladderCancer, #blcsm
Introduction
Thorough pelvic lymph node dissection (PLND) is an essential part of surgical treatment for patients with muscle-invasive or refractory non-muscle-invasive bladder cancer. Consensus exists on performing PLND, although its extent remains controversial [1–3]. A growing body of evidence suggests that lymph node (LN) clearance at the time of radical cystectomy (RC) has important prognostic and therapeutic benefits [4–6]. About 25% of patients with muscle-invasive bladder cancer have LN metastases at the time of RC and 45% of patients with T3 or T4 disease harbour nodal disease [7,8]. Adequate PLND has important diagnostic (appropriate staging) and therapeutic (removal of micrometastatic disease and identifying candidates for adjuvant chemotherapy) implications. LN metastasis remains an adverse prognostic indicator of bladder cancer [8].
The method and technique of PLND varies widely among surgeons and institutions [9]. Surgeon’s preference remains the main determinant of the extent and thoroughness of PLND. Other factors that may affect the decision for PLND include patient age, comorbidities, peripheral vascular disease and risk for thromboembolism [10]. Studies on the assessment of adequacy of PLND are limited by their retrospective nature, non-standardisation of templates, variation among surgeons, and selection bias. LN count has been suggested as a surrogate to assess adequacy of PLND at RC. However, various factors in addition to the thoroughness of LN clearance may influence LN count, such as method of submission (separate vs en bloc), technique for histopathological processing, and thoroughness of pathological examination [11,12].
Whatever the template or the level of LN dissection used, the final operative view of the surgical field remains the best quality measure for thoroughness of PLND. However, no objective method currently exists to measure completeness of PLND. We sought to develop and validate an intraoperative structured tool for assessment of the thoroughness and completeness of PLND during RC, Pelvic Lymphadenectomy Assessment and Completion Evaluation (PLACE), that may serve as an intraoperative quality measure for standardising performance and help facilitate training.
Patients, Subjects and Methods
Content Development and Validation
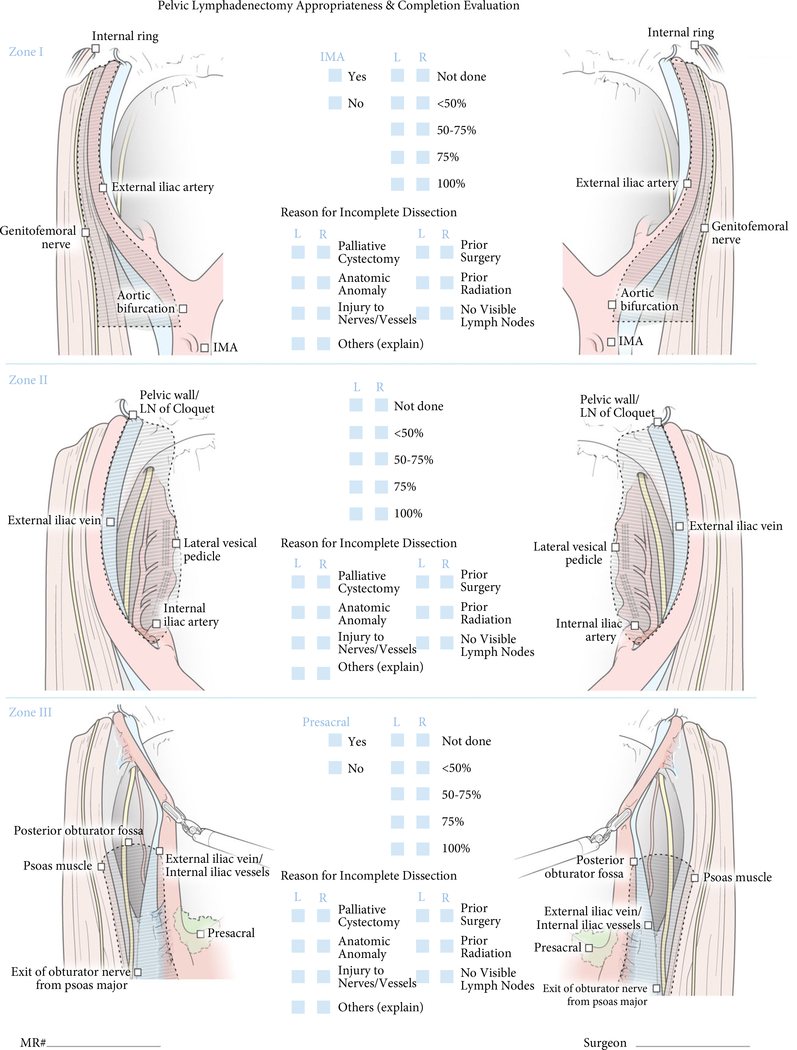
A panel of 11 experts developed a consensus-based structured intraoperative scoring system for completeness of PLND. The expert panel included open and/or robotic surgeons, who were requested to complete a survey about their demographics, operative experience, and perception of the definition of each PLND template (Table 1). The PLND template was divided into three zones that covered a complete PLND template. Zone I was bounded cranially by the aortic bifurcation, laterally by the genitofemoral nerve, medially by the external iliac artery and caudally by the internal ring. Dissection up to the inferior mesenteric artery was included as part of Zone I. Zone II was bounded cranially by the internal iliac artery, laterally by the external iliac vein, medially by the lateral vesical pedicle, and caudally by the lymph node of Cloquet. Dissection of the pre-sacral LNs was considered a part of Zone II. Zone III defined the triangle of Marcille, which is identified after medial retraction of the external iliac artery. It was bounded by psoas muscle laterally, iliac vessels medially, exit of the obturator nerve from psoas major cranially, and the posterior obturator fossa caudally. An illustrative representation was provided for each zone to ensure standardisation and elimination of variation among raters with what defines each zone (Fig. 1). Raters were allowed to provide the reasons for incomplete PLND (e.g. palliative cystectomy, prior surgery, irradiation, etc.).
Table 1.
Demographics and experience of the expert panel.
| Variable | n/N |
|---|---|
| Age, years | |
| 40–55 | 8/11 |
| >55 | 3/11 |
| Urology practice, years | |
| 5–10 | 4/11 |
| 10–15 | 3/11 |
| >15 | 4/11 |
| Formal MIS training | 6/11 |
| RAS experience, n | |
| <50 | 2/11 |
| 50–100 | 1/11 |
| 100–150 | 1/11 |
| >150 | 7/11 |
| Open RC experience, n | |
| <50 | 4/11 |
| 50–100 | 3/11 |
| 100–150 | 0/11 |
| >150 | 4/11 |
| Laparoscopic RC experience, n | |
| <50 | 11/11 |
| RARC experience, n | |
| <50 | 6/11 |
| 50–100 | 2/11 |
| 100–150 | 1/11 |
| >150 | 2/11 |
| Perform PLND routinely during RC | 11/11 |
| Template of PLND | |
| Standard | 1/11 |
| Extended | 10/11 |
| Routinely remove presacral LNs | 3/11 |
| Routinely remove IMA LNs | 1/11 |
MIS, minimally invasive surgery; RAS, robot-assisted surgery; IMA, inferior mesenteric artery.
Fig. 1.
Pelvic Lymphadenectomy Appropriateness and Completion Evaluation (PLACE) scoring.
Reliability
Reliability measures the ability of PLACE to yield consistent results when applied by different raters. In all, 21 de-identified console feed videos of robot-assisted bilateral extended PLND were assessed by the 11 experts using PLACE to determine the inter-rater reliability (IRR) of the assessment tool. The videos were edited to show the initial and final views of PLND. Raters were blinded about the extent of PLND, as well as the operator identity.
LN Clearance
Lymph node clearance was defined as the proportion of LNs cleared from each zone using PLACE, where a perfect PLND established a score of 600 (100% × 3 zones × 2 sides). A score of 800 was the maximum possible when super-extended PLND was performed. We further investigated the correlation between LN clearance and LN count.
Concurrent Validation
Pelvic Lymphadenectomy Appropriateness and Completion Evaluation was compared to the current method of assessment of PLND (depending on the templates and LN count). Extended PLND was performed in 18 consecutive patients prospectively using PLACE and compared with our retrospective series performed using the extended template for LN count, operative time, estimated blood loss (EBL), and complications.
Statistical Analysis
Data were summarised using descriptive statistics. IRR of PLACE among the 11 raters was assessed using Kendall’s coefficient. Spearman correlation was used to test the correlation between LN clearance and LN count. Perioperative outcomes and LN count were compared between patients who received PLND assessed by PLACE and the remaining patients using the chi-squared test for categorical and Wilcoxon rank-sum test for ordinal data. Statistical significance was set at alpha level 0.05. All statistical analyses were performed using Statistical Analysis System (SAS®) 9.3 (SAS Institute, Cary, NC, USA).
Results
Content Development and Validation
The structure and content of PLACE was developed by the expert panel. There was a general agreement among the expert panel on the landmarks that constitute each template except for three landmarks for the standard, two for the extended and one for the super-extended templates (Table 2).
Table 2.
Templates defined by the expert panel.
| Landmarks | Consensus, n |
||
|---|---|---|---|
| Standard | Extended | Super-extended | |
| Obturator | 11 | 11 | 11 |
| Hypogastric | 11 | 11 | 11 |
| External iliac | 11 | 11 | 11 |
| Common iliac | 9 | 11 | 11 |
| Aortic bifurcation | 4 | 10 | 11 |
| Presacral | 2 | 6 | 11 |
| Inferior mesenteric artery | 0 | 0 | 9 |
Reliability
A significant reliability was achieved for all PLACE zones among the 11 raters for the 21 PLND videos. The highest agreement was for PLACE zones I and III (Table 3). As the videos included only extended PLNDs, assessment of dissection of pre-sacral and proximal extent to the inferior mesenteric artery was not possible.
Table 3.
Inter-rater reliability (IRR) among the 11 experts for Pelvic Lymphadenectomy Assessment and Completion Evaluation (PLACE) zones.
| Zone | Kendall’s coefficient | P |
|---|---|---|
| Zone I (Left) | 0.49 | <0.01 |
| Zone I (Right) | 0.33 | <0.01 |
| Zone II (Left) | 0.36 | <0.01 |
| Zone II (Right) | 0.22 | <0.01 |
| Zone III (Left) | 0.55 | <0.01 |
| Zone III (Right) | 0.35 | <0.01 |
LN Clearance
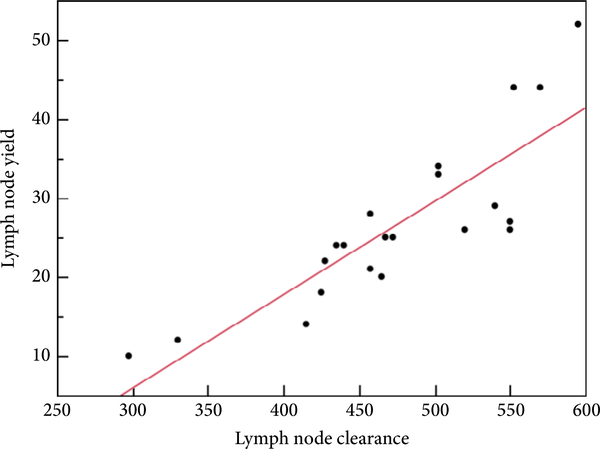
The median (interquartile range) LN clearance was 468 (431–545). There was a significant positive correlation between LN clearance and LN count (R2 = 0.70, P < 0.01; Fig. 2).
Fig. 2.
Correlation between lymph node (LN) clearance score and LN count.
Concurrent Validation
Baseline patient and disease characteristics were comparable between both groups. There was no significant difference between groups for perioperative outcomes except that the No PLACE group had longer mean operative times (360 vs 225 min, P < 0.001) and less patients had an EBL of <500 mL (69% vs 100%, P = 0.04). PLACE yielded a higher mean LN count when compared to No PLACE but the difference was not statistically different when analysed as either as a continuous or categorical variable. There was also no significant difference in 30- and 90-day complications (Table 4).
Table 4.
Perioperative outcomes using Pelvic Lymphadenectomy Assessment and Completion Evaluation (PLACE) vs no PLACE.
| Variable | No PLACE, n (%) | PLACE, n (%) | P |
|---|---|---|---|
| N | 432 (96) | 18 (4) | |
| Age, years, mean | 69 | 69 | 0.90 |
| BMI, kg/m2, mean | 29 | 27 | 0.12 |
| ASA score, median | 3 | 3 | 1.00 |
| ASA score ≥3 | 227 (57) | 18 (100) | <0.001 |
| Prior surgery | 226 (55) | 11 (61) | 0.60 |
| Operative time, min, median | 360 | 225 | <0.001 |
| EBL <500 mL | 298 (69) | 10 (100) | 0.04 |
| ≥pT3 | 183 (46) | 7 (37) | 0.44 |
| pN positive | 102 (24) | 4 (27) | 0.78 |
| PSM | 44 (10) | 1 (7) | 0.66 |
| LN count ≥20 | 251 (58) | 10 (77) | 0.17 |
| LN count, mean | 22 | 29 | 0.13 |
| 30-day complications | 190 (46) | 11 (61) | 0.21 |
| 90-day complications | 241 (58) | 13 (72) | 0.24 |
BMI, body mass index; ASA, American Society of Anesthesiologists; PSM, positive soft tissue surgical margins; probabilities in bold are statistically significant.
Discussion
The presence of LN metastasis is associated with poor recurrence-free and overall survival [8]. Adequate PLND is crucial for appropriate staging, removal of micrometastatic disease, and identifying candidates for adjuvant chemotherapy. Even in histologically LN-negative disease, PLND is crucial as micrometastatic disease has been detected using reverse transcriptase-PCR in up to one-third of patients [13,14]. Despite the clear prognostic and therapeutic significance of PLND, surgical approach varies by anatomical extent (quantity), and thoroughness (quality) of dissection. Prior reports, similar to our present study, demonstrated variation among surgeons and institutions on the extent as well as the boundaries of dissection templates, which may account in part for the wide variation in LN counts even within a defined anatomical template [3,11,15]. Whatever the template used, there is lack of objective assessment and quality control for what defines quality PLND. We developed and validated PLACE as a structured and objective tool for intraoperative assessment of the thoroughness and completeness of PLND. PLACE divides PLND into well-defined zones with measurable outcomes to provide a standardised means to measure and report the quality of PLND. In this context, PLND performed by residents and fellows can be assessed in real-time by the attending surgeons using the PLACE scoring sheet.
Pelvic Lymphadenectomy Appropriateness and Completion Evaluation has been shown to yield consistent results when used by 11 raters to evaluate the three PLND zones. The strongest agreement was for PLACE zones I and III. Variation in what defines technical proficiency among fully trained specialty surgeons has been demonstrated previously [16]. Assessment of surgical performance using video recordings provides an adequate and reliable measure for technical proficiency [17]. As the adequacy of PLND and the number of positive LNs influence survival, development of a clinically relevant intraoperative assessment tool for the adequacy of PLND is crucial as a quality control tool. The surgeon can refer to the scoring sheet to modify his procedure. We found a positive correlation between LN clearance based on PLACE and the LN count. PLACE was not inferior to current practice, and in addition provides a real-time intraoperative assessment of PLND. LN count has been used as a surrogate measure for adequacy of PLND, despite it varying widely among institutions and pathologists [18]. The anatomical extent of dissection, the method of submission for pathological analysis (packets vs en bloc), and the histological processing and examination used by the pathologist techniques to identify LNs vary widely [11,12,19]. A less measurable factor, the thoroughness of dissection, is critical but has been difficult to study. The lack of intraoperative assessment of LN count is another deficiency. Therefore, LN count cannot be used for quality control in real-time during surgery. PLACE provides a reliable tool for intraoperative assessment and quantification of the completeness and appropriateness of PLND. Shorter operative times and lower EBL encountered with PLACE may be explained by a more systematic performance of PLND while using PLACE, or simply due to the increased experience with robot-assisted radical cystectomy (RARC), as these cases were performed recently after the learning curve had been achieved. There was no significant difference in terms of complications between both groups.
The number of RCs performed with robot assistance rose dramatically from <1% in 2004 to 13% in 2010 [20], but concerns remain about the feasibility of achieving adequate LN clearance [21]. A high extended template dissection up to the inferior mesenteric artery has been shown to be feasible using a robot-assisted approach [21]. Although not investigated in the present study, the structure of PLACE follows the principles of modular training, which can facilitate training of residents and fellows. PLND was divided into well-defined and measurable portions, so that performance can be quantified and technical proficiency of trainees can be assessed and progress monitored. PLACE can provide constructive feedback on the performance of individual zones. The illustrations provided may also decrease variation among raters.
The present study is unique but limitations exist. What represents adequate extent of PLND (up to the aortic bifurcation or up to the inferior mesenteric artery) remains controversial. Our present study does not address this issue, but defines adequate and thorough PLND that can be used with any extent of template. Videos were all for extended PLND at a single institution, which limits the ability to draw conclusions about different templates. Prospective evaluation was performed on only a few patients. The lack of video performances by surgeons of variable experience prevented testing the constructive validity of the tool. It should be highlighted that PLACE was designed to measure the completeness of PLND only. However, not just the completion is important; the technique is equally important (appropriate use of fourth arm, avoidance of bleeding and injury of important structures). Future studies will compare whether PLACE affects operative times, intraoperative complications, incidence of lymphocoeles, intestinal obstruction, and the LN count.
In conclusion, PLACE is a structured intraoperative scoring system that can be used to measure and quantify PLND to help facilitate training during RARC.
Abbreviations:
- EBL
estimated blood loss
- IRR
inter-rater reliability
- LN
lymph node
- PLND
pelvic lymph node dissection
- RARC
robot-assisted radical cystectomy
Footnotes
Conflicts of Interest
Dr. Abaza has a research grant from Intuitive Surgical.
References
- 1.Skinner DG. Management of invasive bladder cancer: a meticulous pelvic node dissection can make a difference. J Urol 1982; 128: 34–6 [DOI] [PubMed] [Google Scholar]
- 2.Leissner J, Hohenfellner R, Thuroff JW, Wolf HK. Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosis. BJU Int 2000; 85: 817–23 [DOI] [PubMed] [Google Scholar]
- 3.Abol-Enein H, El-Baz M, Abd El-Hameed MA, Abdel-Latif M, Ghoneim MA. Lymph node involvement in patients with bladder cancer treated with radical cystectomy: a patho-anatomical study — a single center experience. J Urol 2004; 172: 1818–21. [DOI] [PubMed] [Google Scholar]
- 4.Dhar NB, Klein EA, Reuther AM, Thalmann GN, Madersbacher S, Studer UE. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol 2008; 179: 873–8 [DOI] [PubMed] [Google Scholar]
- 5.Poulsen AL, Horn T, Steven K. Radical cystectomy: extending the limits of pelvic lymph node dissection improves survival for patients with bladder cancer confined to the bladder wall. J Urol 1998; 160: 2015–20 [PubMed] [Google Scholar]
- 6.Herr HW, Donat SM. Outcome of patients with grossly node positive bladder cancer after pelvic lymph node dissection and radical cystectomy. J Urol 2001; 165: 62–4 [DOI] [PubMed] [Google Scholar]
- 7.Leissner J, Ghoneim MA, Abol-Enein H et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J Urol 2004; 171: 139–44 [DOI] [PubMed] [Google Scholar]
- 8.Stein JP, Cai J, Groshen S, Skinner DG. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: concept of lymph node density. J Urol 2003; 170: 35–41 [DOI] [PubMed] [Google Scholar]
- 9.Konety BR, Joslyn SA, O’Donnell MA. Extent of pelvic lymphadenectomy and its impact on outcome in patients diagnosed with bladder cancer: analysis of data from the Surveillance, Epidemiology and End Results Program data base. J Urol 2003; 169: 946–50 [DOI] [PubMed] [Google Scholar]
- 10.Hellenthal NJ, Hussain A, Andrews PE et al. Lymphadenectomy at the time of robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. BJU Int 2011; 107: 642–6 [DOI] [PubMed] [Google Scholar]
- 11.Bochner BH, Cho D, Herr HW, Donat M, Kattan MW, Dalbagni G. Prospectively packaged lymph node dissections with radical cystectomy: evaluation of node count variability and node mapping. J Urol 2004; 172: 1286–90 [DOI] [PubMed] [Google Scholar]
- 12.Bochner BH, Herr HW, Reuter VE. Impact of separate versus en bloc pelvic lymph node dissection on the number of lymph nodes retrieved in cystectomy specimens. J Urol 2001; 166: 2295–6 [PubMed] [Google Scholar]
- 13.Marin-Aguilera M, Mengual L, Burset M et al. Molecular lymph node staging in bladder urothelial carcinoma: impact on survival. Eur Urol 2008; 54: 1363–72 [DOI] [PubMed] [Google Scholar]
- 14.Tarin TV, Power NE, Ehdaie B et al. Lymph node-positive bladder cancer treated with radical cystectomy and lymphadenectomy: effect of the level of node positivity. Eur Urol 2012; 61: 1025–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dangle PP, Bahnson RR, Pohar KS. How close are we to establishing standards of lymphadenectomy for invasive bladder cancer? Ther Adv Urol 2009; 1: 167–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abboudi H, Khan MS, Guru KA et al. Learning curves for urological procedures: a systematic review. BJU Int 2014; 114: 617–29 [DOI] [PubMed] [Google Scholar]
- 17.Birkmeyer JD, Finks JF, O’Reilly A et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013; 369: 1434–42 [DOI] [PubMed] [Google Scholar]
- 18.Koppie TM, Vickers AJ, Vora K, Dalbagni G, Bochner BH. Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer 2006; 107: 2368–74 [DOI] [PubMed] [Google Scholar]
- 19.Parkash V, Bifulco C, Feinn R, Concato J, Jain D. To count and how to count, that is the question: interobserver and intraobserver variability among pathologists in lymph node counting. Am J Clin Pathol 2010; 134: 42–9 [DOI] [PubMed] [Google Scholar]
- 20.Leow JJ, Reese SW, Jiang W et al. Propensity-matched comparison of morbidity and costs of open and robot-assisted radical cystectomies: a contemporary population-based analysis in the United States. Eur Urol 2014; 66: 569–76 [DOI] [PubMed] [Google Scholar]
- 21.Desai MM, Berger AK, Brandina RR et al. Robotic and laparoscopic high extended pelvic lymph node dissection during radical cystectomy: technique and outcomes. Eur Urol 2012; 61: 350–5 [DOI] [PubMed] [Google Scholar]