Figure 3.
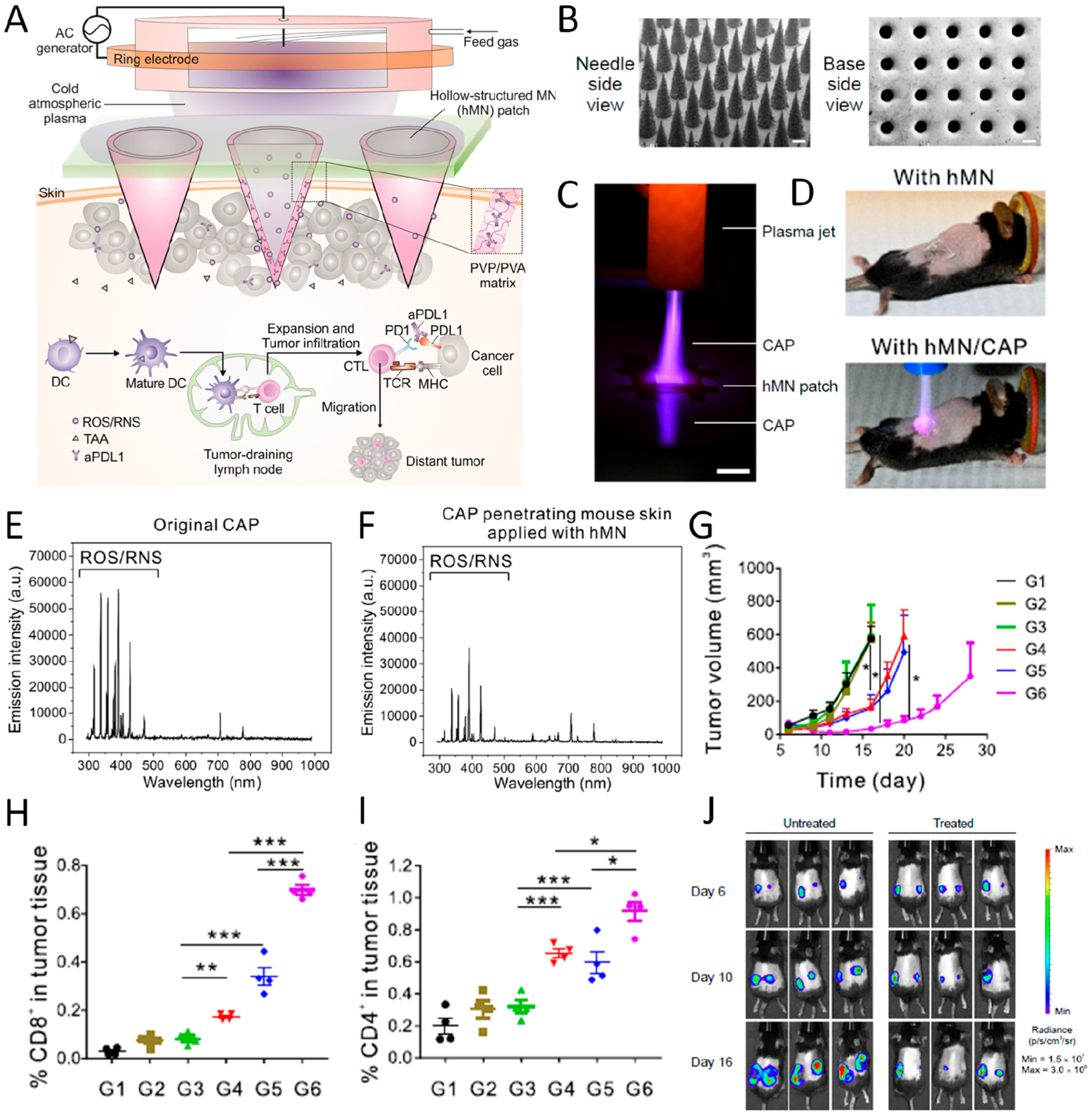
(A) Illustration of hollow-structured microneedle (hMN)-mediated cold atmospheric plasma (CAP) and ICB therapy. TAA, tumor-associated antigen; PVP, poly(vinyl pyrrolidone); PVA, poly(vinyl alcohol). (B) SEM images of MNs from top and bottom side (scale bar 200 μm). (C) Photograph of CAP penetrating through hMN (scale bar 1 cm). (D) Images of mice treated with hMN and hMN combined with CAP therapy. (E, F) Optical emission spectrum of original CAP and CAP penetrating mouse skin applied with hMN. (G) Tumor growth curve of mice with different treatments including untreated (G1) and treated with CAP (G2), CAP with solid-structured microneedle (sMN) (G3), CAP with hMN (G4), hMN loaded with aPD-L1 (G5), and CAP with hMN loaded with aPD-L1 (G6). (H, I) Percentage of CD8+ and CD4+ T cells in tumor 3 days after treatment. Data are presented as mean ± SEM. (J) Bioluminescence image of untreated mice and mice treated with CAP and hMN-aPD-L1 in a distant tumor model. Data are presented as mean ± SEM *P < 0.05; **P < 0.01; ***P < 0.001. Reprinted and modified with permission from ref 3. Copyright 2020 National Academy of Sciences.
