Figure 4. HdrM antagonizes HdrR via membrane sequestration.
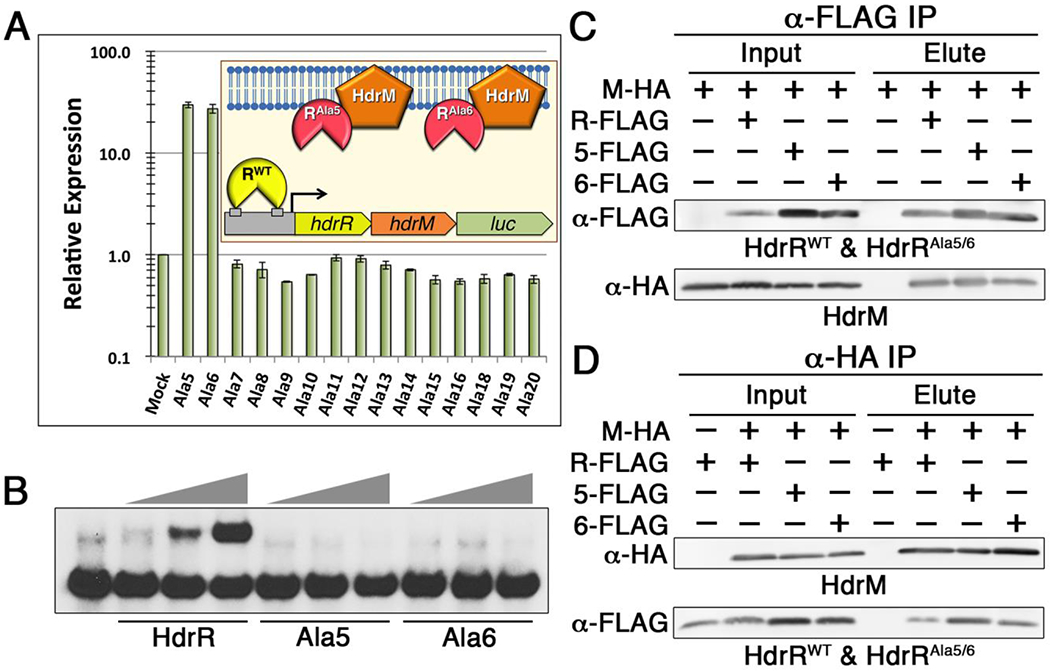
A) A series of DNA binding-defective alanine mutants of HdrR was ectopically expressed to identify mutant proteins capable of activating an hdrRM luciferase reporter. As illustrated in the graph inset, activation of the reporter strain is indicative of competitive inhibition of the wild-type HdrR/M interaction. Based upon the greatly increased luciferase activity triggered by ectopic expression of the Ala5 and Ala6 mutants, both were identified as candidate competitive inhibitors. Data are presented relative to the parent reporter strain (Mock), which was arbitrarily assigned a value of 1. The presented results represent the means of three independent experiments ± SD. B) EMSA of the hdrRM operon promoter region was used to compare the DNA binding affinities of the wild-type HdrR vs. that of the Ala5 and Ala6 HdrR mutants. All EMSA reactions contained a range of 10 – 30 μg of protein incubated with 1 ng of labeled hdrRM promoter region DNA probe. C) Coimmunoprecipitation was used to detect heteromeric complex formation between HA epitope tagged HdrM (M-HA) and FLAG epitope tagged HdrR (R-FLAG), the HdrR Ala5 mutant (5-FLAG), and the HdrR Ala6 mutant (6-FLAG). Samples were immunopurified with α-FLAG affinity resin and the results are shown using both α-FLAG and α-HA western blots. Elution samples were loaded in the same order as the input samples. D) The same epitope tagged proteins were immunopurified with α-HA affinity resin. Elution samples were loaded in the same order as the input samples.
