ABSTRACT
Derris scandens, Albizia procera, and Diospyros rhodocalyx have traditionally been used as herbal remedies for pain relief in Thailand. The ethanolic extracts of these plants obtained by Soxhlet extraction were analyzed by the developed high-performance liquid chromatography-diode-array detection method. Lupeol, the anti-inflammatory triterpene, was selected as a chemical marker for this investigation. All extracts together with that compound were further evaluated for their potential on anti-inflammatory activity using 5-lipoxygenase inhibition assay. Lupeol in each extract was quantified and expressed in the range of 21.44 ± 0.89–40.72 ± 0.40 mg per 100 g of crude drug and the enzyme inhibitory activity of all tested extracts presented as half-maximal inhibitory concentration values ranged between 63.71 ± 2.09 and 91.09 ± 1.40 μg/mL. This study shows that the developed analytical method is effective for analyzing triterpene lupeol in these plants and also reveals the relationship between a lupeol content and the anti-inflammatory effect.
Keywords: Albizia procera, anti-inflammatory activity, Derris scandens, Diospyros rhodocalyx, lupeol
INTRODUCTION
Derris scandens (DS) (Roxb.) Benth. is found throughout Asia. In Thailand, it has been used traditionally for pain treatment.[1] Phytochemical studies of the plant have shown that its stems contain various bioactive compounds, such as flavonoids and terpenoids.[2] Among these compounds, the isoflavone genistein was determined to exhibit anti-inflammatory activity.[3] The compound has been assigned as a marker for the chemical analysis of DS extract in Thai Herbal Pharmacopoeia since 2017.[4] Although genistein was affirmed to possess the pharmacological effects that contribute to traditional use as pain relievers, the other chemical constituents that can be isolated from DS stems are also interesting to researchers exploring their related bioactivities. Among the interesting active principles, lupeol, a pentacyclic triterpene, has been investigated for its potential utilization as an alternative chemical marker in the quality assessment of plants. Lupeol is found in many medicinal plants, and its anti-inflammatory activity has been a topic of focus.[5,6] With this background, lupeol was proposed for exploration in the present study that the quantitative analysis of lupeol in DS materials would be established. Furthermore, two other medicinal plants were also examined in this study, including Albizia procera (AP) (Roxb.) Benth. and Diospyros rhodocalyx (DR) Kurz., which have been used traditionally in Thailand as important ingredients in several herbal recipes for pain relief.[7,8] According to the previous reports of these plants on anti-inflammatory activity,[7,8,9] we hypothesized that the active extracts of these plants might contain lupeol as one of their chemical constituents. Thus, chemical analysis was performed using Soxhlet extraction and high-performance liquid chromatography-diode-array detection (HPLC-DAD) in order to examine the content of lupeol in these plants. Moreover, the inhibition assay on 5-lipoxygenase (LOX) enzyme was conducted to evaluate the performance of the plant extracts on anti-inflammation.
MATERIALS AND METHODS
Chemicals and materials
The stems of DS and the barks of AP and DR were purchased from a traditional Thai pharmacy in July 2020. They were authenticated by comparing with genuine plant materials deposited at the Department of Pharmacognosy, College of Pharmacy, Rangsit University, Thailand. The verification of these crude drugs was performed using techniques that have been detailed in Thai Herbal Pharmacopoeia.[4] The authenticated specimens were coded as RSU 0089, RSU 0092, and RSU 0093, respectively. Lupeol (99% purity) was supplied from Nanjing Spring and Autumn Biological Engineering Co., Ltd. All analytical reagents were supplied from Honeywell Burdick and Jackson®. All disposable accessories used for the HPLC instrument were procured from S. N. P. Scientific Co., Ltd.
Preparation of sample solutions
DS, AP, and DR were powdered and mixed separately. Each sample was weighed accurately (5.0 g) into a thimble of Soxhlet apparatus. Extraction was achieved using 300 mL of ethanol for 3 h. Besides, the powders of these plants were portioned and mixed equally for creating a combination formula. This mixture was weighed (15.0 g) and extracted in the same way as each crude drug. The ethanolic extract was evaporated to dryness in a rotary evaporator. The concentrated extract of each sample was reconstituted with small amounts of ethanol, further diluted with the same solvent, and sequentially adjusted to furnish the sample solution in a 10 mL volumetric flask.
Preparation of standard solutions
Lupeol (25 mg) was dissolved in methanol and further adjusted in a 50 mL volumetric flask to provide a stock standard solution of 500 μg/mL. The stock standard solution was diluted sequentially with methanol to enable working standard solutions at concentration levels of 10, 20, 50, 100, 200, and 400 μg/mL.
Apparatus and chromatographic conditions
The chromatographic method was carried out using an HPLC instrument (1260 Infinity Series, Agilent Technologies), which was equipped with a photodiode array detector (DAD). The devices were operated using OpenLab ChemStation software. The sample and working standard solutions were filtered through a 0.45 μm nylon membrane before analysis. Each filtered sample (20 μL) was injected into the HPLC system and was separated on Accucore™ XL C18 packed column (250 mm × 4.6 mm i. d., 4 μm) using the isocratic elution of the mobile phase that comprised methanol and acetonitrile in the ratio of 90:10. A flow rate of the mobile phase was adjusted to 1.0 mL/min, and the column chamber of the HPLC instrument was controlled at room temperature. A chromatogram of the analyzed samples was recorded by DAD, and the wavelength of 210 nm was selected to collect the absorbance data for the lupeol compound. The total time for analysis of each sample on the HPLC system was assigned within 12 min.
Method validation
The accuracy and precision of the developed analytical method were investigated using a standard spiked technique by adding a known amount of lupeol (80%–120%w/w) to the extracted samples of the mixture in triplicate. The results were calculated in terms of the percentage of recovery and relative standard deviation. Analyses were performed to investigate the intra- and interday precision. The robustness of the analytical method was evaluated by adjusting the proportions of a mobile phase. The linearity, the lowest concentration of quantification, and the lowest concentration of detection were also examined following the guideline revealed in a previous report.[10]
Quantification of lupeol in extracts
The amount of lupeol was calculated using a linear regression equation of the calibration curve of working standard solutions. Lupeol contents in each sample were examined in triplicate and displayed as milligrams per 100 g of the crude drug. The contents were exhibited together with their SD values. The difference of lupeol contents among the sample groups was examined using one-way analysis of variance (ANOVA) with Tukey's multiple comparison post hoc test (PSPP, GNU Project) and a value of P < 0.05 determined for the statistical significance of all tested groups.
5-Lipoxygenase inhibition assay
A 5-LOX inhibition assay was performed to assess the anti-inflammatory activity of the plant extracts, including DS, AP, DR, and the mixture. Lupeol, a marker in the quantitative analysis, was also put through the test. The assay was conducted using the spectrophotometric method, as described in the literature.[11] One milliliter of 0.1 M phosphate buffer pH 9.0 containing 10 μL of 5-LOX enzyme (7.9 U/mL) and 20 μL of a series of the dilutions of tested samples (5–300 μg/mL) were dissolved in methanol and then incubated at room temperature for 10 min. Twenty-five microliters of 62.5 μM sodium linoleate was added to introduce the enzyme activity. The reaction kinetics was observed at a wavelength of 234 nm using a microplate reader (BioTek Synergy HT). The early reaction rates were proposed from the slope of the strength-line portion of the curve, and the inhibition of enzyme activity was calculated from three independent experiments by comparison with the control (methanol). The results of the 5-LOX inhibition assay for all tested samples were expressed as half-maximal inhibitory concentration (IC50) values. The intensity of enzyme inhibition for the tested extracts and lupeol was compared with indomethacin, a nonsteroidal anti-inflammatory drug, which was used as a positive control. The statistical significance of the IC50 values among the tested samples was considered at a value of P < 0.05.
RESULTS AND DISCUSSION
Method validation
All tested parameters of the validation displayed appreciable values, as summarized in Table 1. In addition, there were no compounds found to interfere with the signal of lupeol in the chromatograms of extracted samples in the area of lupeol peak at the retention time of 9.3 min, as shown in Figure 1. Thus, the method was specified and effective for the determination of lupeol in these plant extracts.
Table 1.
Validation data for the high-performance liquid chromatography analysis of lupeol
| Parameters | Results |
|---|---|
| Linear range, µg/ml | 10-400 |
| Correlation coefficient, r2 | 0.9998 |
| Precision, % RSD | |
| Intraday (n=9) | 0.64-1.21 |
| Interday (n=9) | 0.79-1.44 |
| Accuracy, % recovery±SD | |
| Intraday (n=3) | |
| 80% level | 96.43±1.02 |
| 100% level | 97.00±0.62 |
| 120% level | 92.45±0.89 |
| Interday (n=3) | |
| 80% level | 94.77±1.33 |
| 100% level | 95.43±1.38 |
| 120% level | 91.86±0.73 |
| Limit of detection, (µg/ml) | 1.50 |
| Limit of quantification, (µg/ml) | 5.00 |
| Robustness, % RSD (methanol:acetonitrile) | |
| 89:11 | 1.07 |
| 90:10 | 0.37 |
| 91:9 | 0.18 |
RSD: Relative standard deviation, SD: Standard deviation
Figure 1.
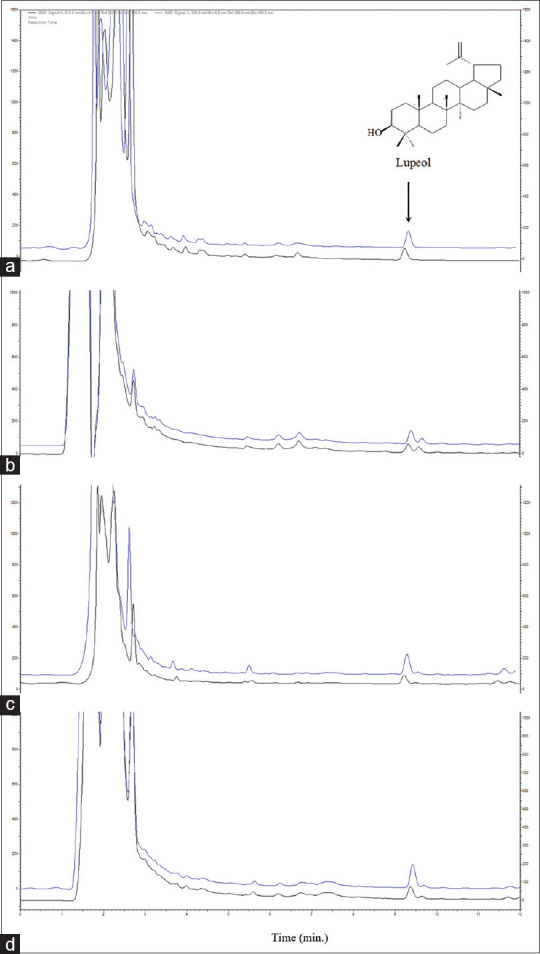
Overlay chromatograms showing the peak for lupeol in a standard-spiked sample (upper line) and a blank sample (bottom line) from different extracts: Derris scandens (a), Albizia procera (b), Diospyros rhodocalyx (c), and the mixture (d)
Lupeol content in different plant extracts
Among different plants, DR possessed the highest yield of lupeol (40.72 ± 0.40 mg/100 g), whereas AP carried the lowest content of lupeol (21.44 ± 0.89 mg/100 g). Lupeol compound was detected in all plant samples and its content was quantified by the developed HPLC-DAD method, as summarized in Table 2. In our investigation, a significant difference of lupeol contents in the extracts of three medicinal plants was observed. One-way ANOVA showed that DS, AP, DR, and the mixture extracts had significant differences in terms of lupeol content (P < 0.05, F = 151.77, df = 3, 8). Tukey's post hoc test further noted that the amount of lupeol among tested samples was particularly different except for the contents between DS and the mixture, which showed no significant difference in the content of that compound between them (P = 0.885), as shown in Figure 2. The yields of lupeol in DS (32.79 ± 0.91 mg/100 g) and the mixture (32.13 ± 1.78 mg/100 g) were observed evenly due to the fact that the quantified lupeol in DS was close to the ideal median value of lupeol in the three subject plants (31.65 mg/100 g). The observed yield of lupeol in the mixture probably corresponded to the theoretical yield that could be calculated from the average lupeol contents in each plant. This observation indicated that lupeol could be used as a practical active marker for the mixture product, which comprised these medicinal plants as ingredients. Further, our results point to the fact that lupeol has sufficient quantity in plants and could possibly be a suitable marker for the chemical analysis of crude drugs and related materials. Referring to the published analytical method for the determination of an active principle in DS and its related herbal formulas, the isoflavone genistein was used as one of the chemical markers for substantiating the plant materials in a previous report.[12] Comparing the signal of the active marker in the HPLC chromatograms of the literature with the peak of triterpene lupeol in the chromatograms of this study, lupeol that was determined by our developed method using an isocratic system was more recognizable. Thus, it could be used effectively as an analytical tool for the quality control of DS, AP, and DR materials. However, the variation of lupeol contents in the analyzed samples might be relevant to the different sources of medicinal plants. These factors might affect the yield of active compounds in plant-related materials. Accordingly, the varying contents of the chemical markers among different herbal sources would be further investigated.
Table 2.
Lupeol content and the anti-inflammatory activity of the extracts
| Sample | Content (mg/100 g) | IC50 (µg/mL) |
|---|---|---|
| DS | 32.79±0.91 | 73.41±1.10 |
| AP | 21.44±0.89 | 91.09±1.40 |
| DR | 40.72±0.40 | 71.06±1.95 |
| Mixture | 32.13±1.78 | 63.71±2.09 |
| Lupeol | - | 69.26±1.39 |
| Indomethacin | - | 62.21±1.07 |
Mean±SD, n=3. SD: Standard deviation, IC50: Half maximal inhibitory concentration, DS: Derris scandens, AP: Albizia procera, DR: Diospyros rhodocalyx
Figure 2.
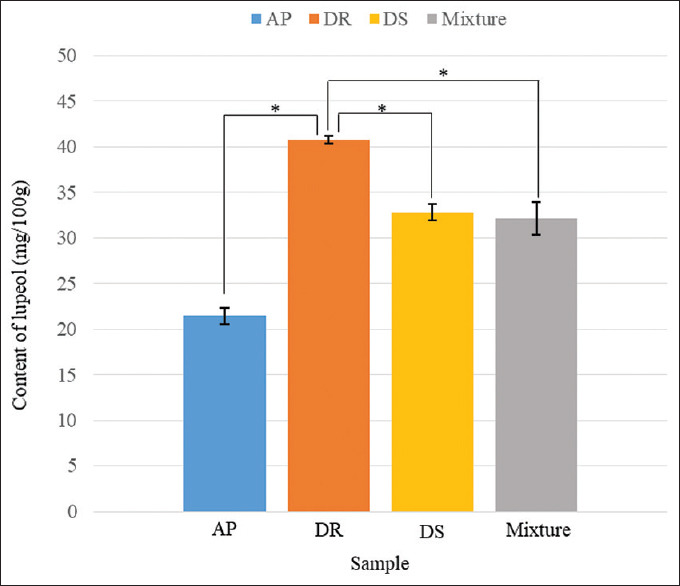
Comparison of a lupeol content in different extracts. Significant difference: P < 0.05 (*)
Anti-inflammatory activity of the plant extracts
The extracts of the crude drugs including DS, AP, and DR showed inhibitory activity with IC50 values of 73.41 ± 1.10, 91.09 ± 1.40, and 71.06 ± 1.95 μg/mL, respectively, as summarized in Table 2. Thus, the order of enzyme inhibitory activity among them was DR > DS > AP.
DR had the highest content of lupeol; it also possessed a strong inhibitory effect compared to DS and AP. On the other hand, the lowest activity was observed in AP, which occupied a lupeol content lower than in the others. Observations indicated that the anti-inflammatory potency of these extracts and their lupeol contents had a relationship and that the observed activity was directly proportional to the lupeol content. Comparing the activity of DS, AP, and DR samples with the mixture that contained DS, AP, and DR uniformly in a formula, the mixture possessed the strongest enzyme inhibitory activity with the IC50 value of 63.71 ± 2.09 μg/mL. Its inhibitory effect was also noted to be higher than lupeol (69.26 ± 1.39 μg/mL). In addition, the statistical difference in the activity between the mixture sample and lupeol compound was significantly noticeable (P < 0.05). Besides, the enzyme inhibitory effects between the mixture and a positive control indomethacin (62.21 ± 1.07 μg/mL) were observed similarly at P < 0.05. Thus, a positive correlation between lupeol contents and the anti-inflammatory effects demonstrated in the DS, AP, and DR samples was not related to the mixture sample. While the content of lupeol in the mixture was quantified similar to that in the DS sample and was lower than the DR sample, the anti-inflammatory activity of the mixture was better compared with the other samples and the lupeol compound. The data suggested that the capability for enzyme inhibition of the mixture seemed to involve the other anti-inflammatory agents that might belong to the chemical constituents of DS, AP, and DR. Phytochemical investigations of DS and AP have demonstrated that these plants contain a high amount of biologically active substances such as genistein, daidzein, and flavonol glycosides.[3,8] These flavonoids were reported to correspond with anti-inflammatory properties in both in vitro and in vivo pharmacological studies.[3,8,9] However, other pentacyclic triterpenes such as betulin and betulinic acid that were found in DR have been reported to exhibit a remarkable property on anti-inflammation.[7,13] Thus, these phytoconstituents might be responsible for the enzyme inhibitory effect of the mixture formula and explain why its anti-inflammatory activity was stronger than lupeol and a comparable positive control in this examination. Our study pointed out that the mixture would be the most efficient formula for an anti-inflammatory herbal recipe. Nevertheless, an exhaustive study on pharmacology and the phytochemical analysis of other promising anti-inflammatory agents in the mixture should be considered and undertaken.
CONCLUSION
This study presents an effective analytical tool for the quantification of lupeol in extracted herbal samples. Such a tool could be applied as an alternative method for the analysis of chemical markers in the quality assessment of these plant-related materials. This exploration is the first report that verified the presence of anti-inflammatory triterpene lupeol in DS, AP, DR, and the mixture formula. The potential of their extracts was also elaborated in order to perform further in-depth studies on anti-inflammatory properties.
Financial support and sponsorship
This study was financially supported by the Research Institute of Rangsit University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank the Research Institute of Rangsit University for providing the necessary facilities.
REFERENCES
- 1.Puttarak P, Sawangjit R, Chaiyakunapruk N. Efficacy and safety of Derris scandens (Roxb.) Benth. for musculoskeletal pain treatment: A systematic review and meta-analysis of randomized controlled trials. J Ethnopharmacol. 2016;194:316–23. doi: 10.1016/j.jep.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Hussain H, Al-Harrasi A, Krohn K, Kouam SF, Abbas G, Shah A, et al. Phytochemical investigation and antimicrobial activity of Derris scandens. J King Saud Univ Sci. 2015;27:375–8. [Google Scholar]
- 3.Laupattarakasem P, Houghton PJ, Hoult JR. Anti-inflammatory isoflavonoids from the stems of Derris scandens. Planta Med. 2004;70:496–501. doi: 10.1055/s-2004-827147. [DOI] [PubMed] [Google Scholar]
- 4.Department of Medical Sciences, Ministry of Public Health. Thai Herbal Pharmacopoeia 2017. Bangkok: The Agricultural Co-operative Federation of Thailand, Ltd; 2017. [Google Scholar]
- 5.Fernández MA, de las Heras B, Garcia MD, Sáenz MT, Villar A. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J Pharm Pharmacol. 2001;53:1533–9. doi: 10.1211/0022357011777909. [DOI] [PubMed] [Google Scholar]
- 6.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285:109–15. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Othong R, Trakulsrichai S, Wananukul W. Diospyros rhodocalyx (Tako-Na), a Thai folk medicine, associated with hypokalemia and generalized muscle weakness: A case series. Clin Toxicol (Phila) 2017;55:986–90. doi: 10.1080/15563650.2017.1330957. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava V, Kumar Verma S, Panwar S, Deep P, Verma S. A brief review on phytopharmacological reports on Albizia procera. Asian J Pharm Pharmacol. 2020;6:144–9. [Google Scholar]
- 9.Sangeetha M, Chamundeeswari D, Saravana Babu C, Rose C, Gopal V. Attenuation of oxidative stress in arthritic rats by ethanolic extract of Albizia procera benth bark through modulation of the expression of inflammatory cytokines. J Ethnopharmacol. 2020;250:112435. doi: 10.1016/j.jep.2019.112435. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal SS, Yallatikar T, Gurjar P. Reversed-phase high-performance liquid chromatographic method development and validation for allyl isothiocyanate estimation in phytosomes of Brassica nigra extract. J Adv Pharm Technol Res. 2019;10:126–31. doi: 10.4103/japtr.JAPTR_382_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashour ML, El-Readi M, Youns M, Mulyaningsih S, Sporer F, Efferth T, et al. Chemical composition and biological activity of the essential oil obtained from Bupleurum marginatum (Apiaceae) J Pharm Pharmacol. 2009;61:1079–87. doi: 10.1211/jpp/61.08.0012. [DOI] [PubMed] [Google Scholar]
- 12.Ayameang O, Rattarom R, Mekjaruskul C, Caichompoo W. Anti-inflammatory activity and quantitative analysis of major compounds of the mixtures of Derris scandens (DZSS) formula. Pharmacogn J. 2020;12:828–34. [Google Scholar]
- 13.Hordyjewska A, Ostapiuk A, Horecka A, Kurzepa J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem Rev. 2019;18:929–51. [Google Scholar]


