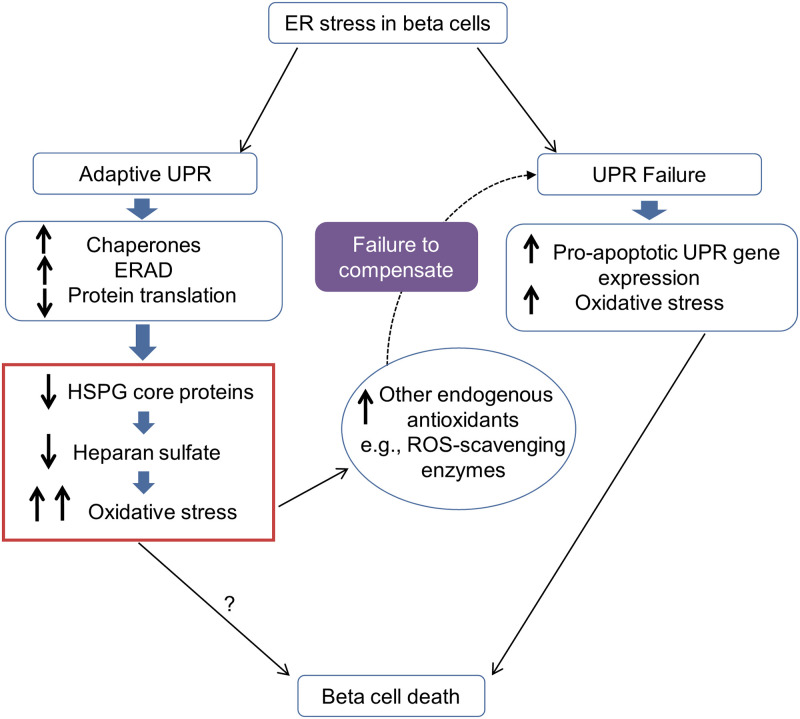
Fig 10. The proposed model: ER stress inhibits the synthesis of HSPG core proteins and HS in T2D-prone beta cells.
In pre-T2D pancreatic beta cells, ER stress initiates the unfolded protein response (UPR) which acts to alleviate the stress by increasing molecular chaperones for protein folding, increasing ERAD and reducing the translation of mRNAs to a range of “general” proteins. As a consequence, HSPG core protein synthesis is impaired, which in turn severely reduces the synthesis of HS, a constitutive non-enzymatic antioxidant in beta cells. Depletion of beta cell HS increases intracellular ROS and elevates oxidative stress. Oxidative stress induces the expression of secondary antioxidant enzymes to help maintain beta cell homeostasis [57]. Failure to compensate for this stress results in UPR failure, the preferential expression of apoptotic genes and beta cell apoptosis/death. ERAD, ER-associated degradation; T2D, Type 2 diabetes; HS, heparan sulfate; HSPG, heparan sulfate proteoglycan; ROS, reactive oxygen species.