Fig. 3. Roles for the assembly factors RsmA and RimP in CDR maturation.
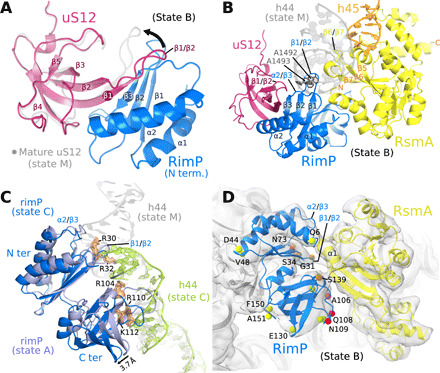
(A) The intermolecular β sheet formed by RimP and uS12, as observed in states A to D (state C shown), superimposed on uS12 of the mature 30S subunit (state M; gray), to highlight the conformational change in the β1/β2 loop of uS12 (arrow). (B) In state B, RimP (blue) and RsmA (yellow) bind adjacent on the 30S subunit and occupy the position of h44 in the mature 30S subunit (gray). (C) In state C, the C-terminal domain of RimP (blue; superposed on state A in violet) withdraws from the h44 binding site, such that the lower part of h44 (green; residues C1409 to G1491) repositions as seen in the mature 30S subunit (gray ribbon), while the upper part and linker region are disordered (not visible in the cryo-EM map). (D) Zoom on the RimP/RsmA interface seen by cryo-EM (state B). Indicated residues (spheres) were observable in the CLEANEX NMR spectrum of free RimP and are colored by the ratio κ of their HN/H2O exchange rates in the absence (kex,free) and presence (kex,+RsmA) of RsmA, κ = kex,free /kex,+RsmA: green (κ = 0.8 to 1.2), orange (κ = 1.6 to 2.0), and red (κ > 2). All RimP residues with significant solvent protection after RsmA addition (κ ≥ 1.6) localize to the RimP/RsmA interface seen in the cryo-EM structure of their 30S complex. See fig. S12 for RimP CLEANEX spectra and derived HN/H2O exchange rates.
