Watch a video presentation of this article
Watch the interview with the author
Answer questions and earn CME
Abbreviations
- AFP
alpha‐fetoprotein
- AUROC
area under the receiver operating characteristics
- CT
computed tomography
- DCP
des‐γ carboxyprothrombin
- ETC
extended Toronto criteria
- 18F‐FDG‐PET
18F‐labeled fluoro‐2‐deoxyglucose positron emission tomography
- HALT
hazard associated with liver transplantation for hepatocellular carcinoma
- HCC
hepatocellular carcinoma
- LDLT
live donor liver transplant
- LT
liver transplantation
- LRT
local‐regional therapy
- MELD
Model for End‐Stage Liver Disease
- MMAT
median Model for End‐Stage Liver Disease at transplant
- MORAL
model of recurrence after liver transplant
- mTOR
mammalian target of rapamycin
- NLR
neutrophil‐to‐lymphocyte ratio
- OPTN
Organ Procurement and Transplantation Network
- RETREAT
risk estimation of tumor recurrence after transplant
- TTV
total tumor volume
- UNOS
United Network for Organ Sharing
- UNOS‐DS
United Network for Organ Sharing/Region 5 down‐staging protocol
Liver transplantation (LT) offers excellent long‐term survival for certain patients with hepatocellular carcinoma (HCC), with mounting evidence that tumor size and number is just one of several factors that predict post‐LT outcome. In this article, we focus on strategies to help refine selection criteria, including incorporating markers of tumor biology and down‐staging to maximize transplant survival benefit. We also will describe how explant‐based recurrence prediction models can be used to personalize post‐LT management.
Selection Criteria
For nonresectable patients with early‐stage HCC, LT remains the optimal treatment strategy with HCC, accounting for more than 25% of all LTs performed in the United States and with an expected 5‐year post‐LT survival rate approaching 80%. Despite being relatively restrictive, the Milan criteria (1 lesion ≤ 5 cm or 2‐3 lesions ≤ 3 cm) remain the benchmark for the selection of transplant candidates with HCC in the United States. In those exceeding the Milan criteria, survival after LT decreases with increasing tumor size and number, although modest expansion can achieve post‐LT survival comparable with the Milan criteria. 1 In an effort to expand access to LT for more patients with HCC while accounting for worldwide organ shortages, selection criteria no longer simply rely on tumor size and number but instead commonly include surrogates of tumor biology. 2 Most notably, high alpha‐fetoprotein (AFP) levels have been consistently identified as a negative predictor of post‐LT outcome independent of tumor burden. 1 , 2 , 3 , 4 , 5 , 6 Additional criteria associated with inferior post‐LT outcome and incorporated into various pre‐LT selection models (Table 1) include elevated des‐γ carboxyprothrombin (DCP) and neutrophil‐to‐lymphocyte ratio (NLR), and positive 18F‐labeled fluoro‐2‐deoxyglucose positron emission tomography (18F‐FDG‐PET) scan. 2
TABLE 1.
Summary of Proposed Pretransplant Selection Models
| Pre‐LT Selection Model | Tumor Burden | Biomarker(s) | Additional Criteria | 5‐Year Post‐LT Overall Survival | AUROC | |
|---|---|---|---|---|---|---|
| US National Policy | Milan or down‐staged to Milan | AFP > 1000 ng/mL reduced to <500 | 80% | |||
| French AFP Model | Size and number (lowest risk: largest tumor ≤ 3 cm and ≤3 tumors) | AFP (lowest risk: ≤100 ng/mL) | 68% if AFP model ≤ 2 versus 47% if AFP model > 2 | 0.7 | ||
| Metro‐Ticket 2.0 | Tumor number + size of largest tumor | AFP | 0.72 | |||
| TTV‐AFP Model | TTV ≤ 115 cm3 | AFP ≤ 400 ng/mL | 75% (at 4 years) for those greater than Milan but within TTV‐AFP | TTV: 0.8 | ||
| ETC | No limit | 1. Bx of largest tumor with poorly differentiated excluded | 68% for those greater than Milan but within ETC | |||
| 2. No cancer‐related sx | ||||||
| Pre‐MORAL | Largest tumor size (lowest risk: ≤3 cm) | AFP (lowest risk: <200 ng/mL) | NLR (lower risk < 5) | 5‐Year recurrence‐free survival: 99% low risk, 70% medium‐risk, 56% high‐risk | 0.82 | |
| HALT‐HCC | Hypotenuse between tumor number and largest tumor size* | lnAFP | MELD‐Na | 0.61 | ||
| MORAL (LDLT) | No limit |
|
83% for those greater than Milan but low MORAL score | 0.84 | ||
| National Cancer Center Korea (LDLT) | Total tumor diameter <10 cm | Negative 18F‐FDG‐PET scan | 84% (versus 60% in those exceeding criteria) | 0.80 | ||
| Kyoto criteria (LDLT) | ≤10 tumors, largest tumor ≤ 5 cm | DCP ≤ 400 mAU/mL | 82% (versus 42% in those exceeding criteria) | Tumor no.: 0.68; size: 0.64; DCP: 0.71 |
Reproduced with permission from Clinical Liver Disease. 2 Copyright 2019, American Association for the Study of Liver Diseases.
By Pythagorean theorem (A2 + B2 = C2); e.g., a patient with three lesions with largest 4 cm would receive tumor burden score of 5.
Abbreviations: Bx, biopsy; lnAFP, natural log AFP; MELD‐Sodium, Model for End‐Stage Liver Disease‐Sodium; sx, symptoms.
The French AFP model 3 and the Metroticket 2.0 model 1 both demonstrate that a combination of AFP and tumor burden parameters predicts post‐LT outcome far better than tumor burden alone. For example, using the Metroticket 2.0 calculator, a patient with a single 7‐cm tumor and AFP of 5 ng/mL would have an excellent 85% predicted 5‐year HCC‐specific survival rate, whereas a patient within Milan criteria with three tumors, largest 3 cm, and an AFP of 200 ng/mL would have only a 70% predicted 5‐year HCC‐specific survival rate. Given the significance of a very elevated AFP, candidates with an AFP > 1000 ng/mL in the United States are not eligible for Model for End‐Stage Liver Disease (MELD) exception until the AFP declines to <500 ng/mL with local‐regional therapy (LRT). 7 Additional recent Organ Procurement and Transplantation Network (OPTN) policy changes include requiring uniform diagnostic criteria with only Liver Imaging Reporting and Data System (LI‐RADS) 5 lesions eligible for priority listing and awarding median MELD at transplant (MMAT)‐3 based on acuity circles after a 6‐month delay both to equalize access to LT for patients with and without HCC and to reduce geographic inequalities. 7
Down‐Staging/Bridging LRT
When expected transplant wait time is >6 months, LRT is typically used as a bridge to control tumor growth and reduce the risk for wait‐list dropout, with tumor progression despite LRT associated with worse post‐LT outcome. 2 , 6 In patients exceeding Milan criteria, LRT can be used to achieve tumor down‐staging, with most published studies using residual tumor within Milan criteria as the endpoint of down‐staging. 8 Successful down‐staging serves as a selection tool for a subset of patients with favorable tumor biology who are likely to do well after LT. To standardize down‐staging criteria, United Network for Organ Sharing (UNOS)/OPTN adopted the UNOS/Region 5 down‐staging protocol (UNOS‐DS; Table 2) in 2017, with patients successfully down‐staged to within Milan criteria eligible to receive automatic MELD exception after the mandatory 6‐month waiting period. 7 Although prospective single‐center and national studies have shown similar post‐LT survival in patients initially meeting UNOS‐DS criteria compared with those always within Milan criteria, 5 , 9 liberalizing down‐staging inclusion criteria results in inferior post‐LT survival. In “all‐comers” initially exceeding UNOS‐DS criteria with no upper limit of tumor size and number, the 3‐year post‐LT survival rate was only 71% compared with 79% in UNOS‐DS patients and 83% in patients with HCC always within Milan criteria. 5 Therefore, “all‐comers” are considered for MELD exception after successful down‐staging only on a case‐by‐case basis. Recommendations for this population are to ensure a longer period of stability before LT to select less aggressive tumors and consider more stringent AFP cutoffs because the 3‐year post‐LT survival rate is only 50% in “all‐comers” with an AFP > 20 ng/mL. 5
TABLE 2.
UNOS Down‐Staging Criteria
| Inclusion Criteria |
|
HCC exceeding Milan criteria but meeting one of the following:
Plus absence of vascular invasion or extrahepatic disease based on cross‐sectional imaging |
| Criteria for Successful Down‐Staging |
|
Residual tumor size and diameter within Milan criteria (1 lesion ≤ 5 cm, 2‐3 lesions ≤ 3 cm)
|
| Criteria for Down‐Staging Failure and Exclusion From LT |
|
| Timing of LT in Relation to Down‐Staging |
|
Despite favorable explant features in a carefully selected down‐staging cohort, 9 successful radiographic down‐staging does not assure actual down‐staging based on explant pathology. Nationally, one‐third of patients with HCC initially meeting UNOS‐DS criteria had been under‐staged with explant tumor beyond Milan compared with <15% in patients always within radiographic Milan criteria. 5 The odds of tumor under‐staging on explant increases by 10% for each 1‐cm increase in total tumor diameter on the last pre‐LT imaging, 5 with under‐staging consistently associated with increased post‐LT HCC recurrence and death. 1 , 4 , 10 Therefore, down‐staging to within Milan criteria should be the minimal requirement for LT, with the recommendation to continue LRT until complete tumor necrosis if sufficient liver function to tolerate additional treatment.
Liver Transplant Survival Benefit
All listed patients with HCC within Milan criteria receive the same allocation priority (i.e., MMAT‐3) regardless of underlying liver function or tumor characteristics. This one‐size‐fits‐all approach seeks to ensure sufficient utility (i.e., post‐LT survival) but largely discounts urgency (i.e., risk for wait‐list dropout). Transplant survival benefit is defined as a patient’s expected post‐LT survival minus their expected survival without LT. Patients with decompensated HCC with MELD score > 13 have a greater risk for wait‐list dropout, and thus increased transplant survival benefit, compared with patients with compensated HCC. 6 One potential solution to improve LT survival benefit would be to account for MELD‐Na score above a certain threshold given increased urgency for LT.
In areas with organ shortages, just as important as avoiding LT in patients with HCC with reduced utility (e.g., AFP > 1000 ng/mL and/or progressive disease despite LRT) is delaying or not pursuing transplant in patients with HCC at low risk for wait‐list dropout. In a national analysis, patients with a single 2‐ to 3‐cm tumor, AFP < 20 ng/mL, and Child’s A cirrhosis with low MELD (12% of the cohort) had a 1‐year probability of dropout of only 5% compared with 20% for all others. 11 This group of patients, especially if they have a complete response to LRT, has a lack of urgency, and thus likely should receive reduced priority for LT.
Posttransplant Management
Even with adherence to the Milan criteria, post‐LT HCC recurrence occurs in approximately 15% of patients and is the most common cause of death in this population. HCC recurrence typically carries a poor prognosis, with <10% of patients eligible for resection and a median survival of approximately 1 year from recurrence diagnosis. The post–model of recurrence after liver transplant (MORAL) score 10 accounts for tumor differentiation, vascular invasion, and tumor number and size on explant and has excellent recurrence prediction (area under the receiver operating characteristics [AUROC], 0.87), although it has not been validated. The risk estimation of tumor recurrence after transplant (RETREAT) score, 4 which has been validated nationally, incorporates AFP at LT, vascular invasion, and the sum of the largest viable tumor diameter (in cm) and number of viable tumors on explant (Table 3). RETREAT stratifies 5‐year recurrence risk rate from <3% in patients without viable tumor on explant and AFP ≤ 20 ng/mL (i.e., RETREAT 0) up to 75% in the highest‐risk patients (RETREAT ≥5) with risk‐based post‐LT surveillance regimen proposed (Table 3). In addition, 3‐year post‐LT survival decreases with increasing RETREAT score: 91% for a score of 0, 80% for a score of 3, and 58% for a score ≥5. 4
TABLE 3.
RETREAT Score Points Table With Corresponding Estimation of HCC Recurrence Risk and Proposed Post‐LT HCC Surveillance Regimen
| Predictor | RETREAT Points |
|---|---|
| AFP at LT (ng/mL) | |
| 0‐20 | 0 |
| 21‐99 | 1 |
| 100‐999 | 2 |
| >1000 | 3 |
| Presence of microvascular invasion | 2 |
| Largest viable tumor diameter + number of viable lesions | |
| 0 (no viable tumor on explant) | 0 |
| 1‐4.9 | 1 |
| 5‐9.9 | 2 |
| ≥10 | 3 |
| RETREAT Score | Recurrence Risk 1 Year After LT | Recurrence Risk 5 Years After LT | Proposed HCC Surveillance Regimen* |
|---|---|---|---|
| 0 | 1% | 3% | No surveillance |
| 1 | 3% | 8% | Every 6 months for 2 years |
| 2 | 4% | 11% | |
| 3 | 5% | 14% | |
| 4 | 11% | 29% | Every 6 months for 5 years |
| ≥5 | 39% | 75% | Every 3‐4 months for 2 years; then every 6 months for years 2‐5 |
Surveillance entails multiphasic CT or magnetic resonance imaging of the abdomen, noncontrast chest CT, and AFP at the recommended interval. This proposed regimen has not been validated.
Currently, there are no proven adjuvant therapies to reduce post‐LT recurrence risk. Because calcineurin inhibitors are associated with increased HCC recurrence and the mammalian target of rapamycin (mTOR) inhibitors appear to have antineoplastic properties, many LT centers convert patients at high risk for recurrence (e.g., RETREAT ≥4) to mTOR inhibitor–based immunosuppression. However, the prospective international phase 3 SiLVER (sirolimus in liver transplant recipients with HCC) trial 12 failed to demonstrate an overall benefit of sirolimus in improving long‐term recurrence‐free survival beyond 5 years after LT.
Conclusion
Figure 1 summarizes the approach to the selection of patients with HCC for LT with criteria no longer simply relying on tumor size and number but instead including surrogates of tumor biology, including AFP and other biomarkers. Recent national HCC policies standardize wait times, exclude LT candidates with AFP > 1000 ng/mL until reduction to <500 with LRT, and grant automatic exception for UNOS‐DS patients who are successfully down‐staged into Milan criteria. Although all wait‐listed patients with HCC receive similar LT priority, overall survival benefit could be improved by accounting for laboratory MELD score and reducing priority for compensated patients with single, small, well‐treated HCC. Finally, individualized explant‐based recurrence risk prediction (e.g., RETREAT) should be used after LT to tailor surveillance and potentially immunosuppression regimens.
FIG 1.
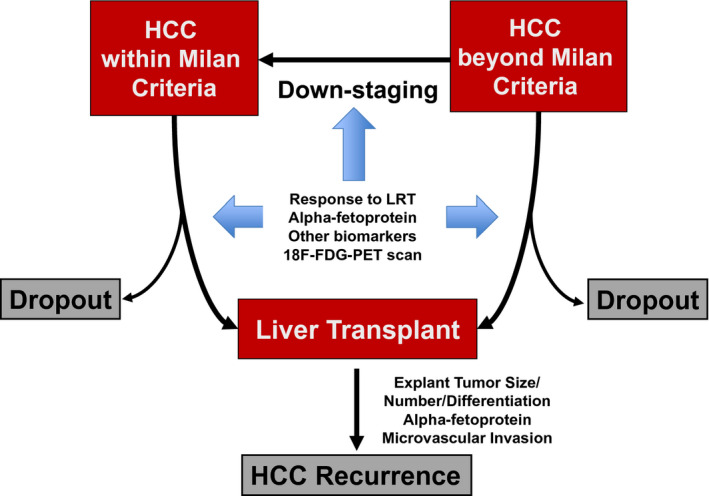
Approach to the selection of patients with HCC for LT and common post‐LT recurrence risk factors. Adapted with permission from Clinical Liver Disease. 2 Copyright 2019, American Association for the Study of Liver Diseases.
Potential conflict of interest: N.M. advises and has received grants from FUJIFILM Wako. He also received grants from Glycotest and TARGET PharmaSolutions.
References
- 1. Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018;154:128‐139. [DOI] [PubMed] [Google Scholar]
- 2. Mehta N, Yao FY. What are the optimal liver transplantation criteria for hepatocellular carcinoma? Clin Liver Dis 2019;13:20‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duvoux C, Roudot‐Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha‐fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986‐994. [DOI] [PubMed] [Google Scholar]
- 4. Mehta N, Dodge J, Roberts JP, et al. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am J Transplant 2018;18:1206‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta N, Dodge JL, Grab JD, et al. National experience on down‐staging of hepatocellular carcinoma before liver transplant: influence of initial tumor burden, alpha‐fetoprotein, and wait time. Hepatology 2020;71:943‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai Q, Vitale A, Iesari S, et al. Intention‐to‐treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology 2017;66:1910‐1919. [DOI] [PubMed] [Google Scholar]
- 7. Organ Procurement and Transplantation Network . Organ Procurement and Transplantation Network (OPTN) Policies. Available at: https://optn.transplant.hrsa.gov/. Accessed August 25, 2020.
- 8. Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: a systematic review and pooled analysis. Liver Transpl 2015;21:1142‐1152. [DOI] [PubMed] [Google Scholar]
- 9. Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long‐term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968‐1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg 2017;265:557‐564. [DOI] [PubMed] [Google Scholar]
- 11. Mehta N, Dodge J, Hirose R, et al. Predictors of low risk for dropout from the liver transplant waiting list for hepatocellular carcinoma in long wait time regions. Am J Transplant 2019;19:2210‐2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geissler EKSA, Zulke C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open‐label phase 3 trial. Transplantation 2016;100:116‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]


