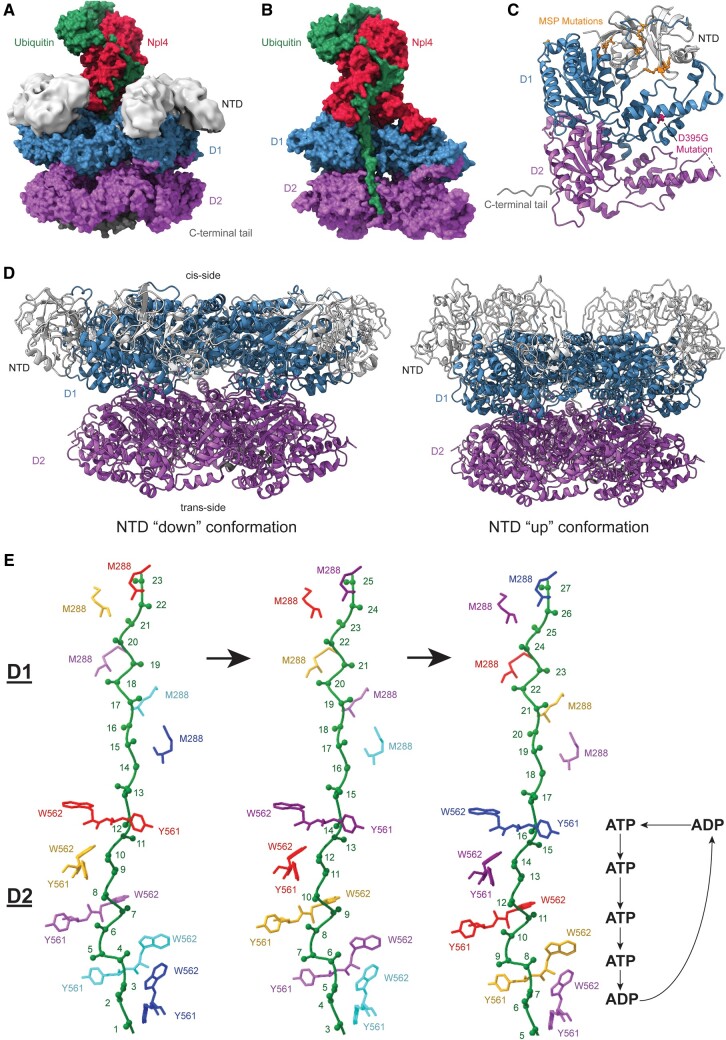
FIGURE 1.
Structures of valosin-containing protein (VCP) processing substrate, its mutations, and different conformational arrangements sampled by VCP during its function. (A) VCP bound to Npl4 (red) and ubiquitinated substrate (green) with N-domains (light gray) modeled in. N-terminal domains (NTD) (light gray) sit on top of the D1 ATPase ring (blue) that sits on top of the D2 ATPase ring (purple). VCP has a C-terminal tail (dark gray) on the trans side of the ring (Atomic structure: PDB 6OA9, N-domains: EMDB 7479). (B) Cut-through of VCP bound to ubiquitinated substrate (green). The substrate is bound to Npl4 (red) on the cis side of VCP and travels through the pore, contacting the D1 ATPase ring (blue) and the D2 ATPase ring (purple) (PDB: 6OA9). (C) Disease-related VCP mutations shown on a VCP monomer. The multisystem proteinopathy (MSP) mutations (orange) are associated with a variety of clinical phenotypes. The MSP mutations generally are near the interface of the NTD (light gray) and the D1 ATPase ring (blue) and may change VCP to have a preference for the “up” conformation. The newly discovered D395G mutation (pink) is associated with vacuolar tauopathy (PDB: 5C19). (D) On the left is the “down” conformation of VCP that has the NTD (light gray) coplanar with the D1 ATPase ring (blue). The “down” conformation can bind some VCP cofactors such as VIMP. The right is the “up” conformation of VCP that has the NTD (light gray) above the D1 ATPase ring (blue). The “up” conformation is required for binding of the Ufd1/Npl4 (UN) cofactors (PDB: 5FTN, 5FTM). (E) The VCP D1 and D2 pore loop staircase processing substrate with hypothesized nucleotide bound states of the D2 pore (D1 nucleotide bound states and how they correlate to D2 nucleotide bound states are still being investigated). Each side chain color represents a monomer that moves down a position in the staircase and eventually returns to the top of the staircase. ATP is bound to all monomers except those at the bottom of the staircase and in process of moving to the top of the staircase. W562 and Y561 are the key aromatic residues in pore loop 2 that bind to substrate in the staircase. (PDB: 6OAB).