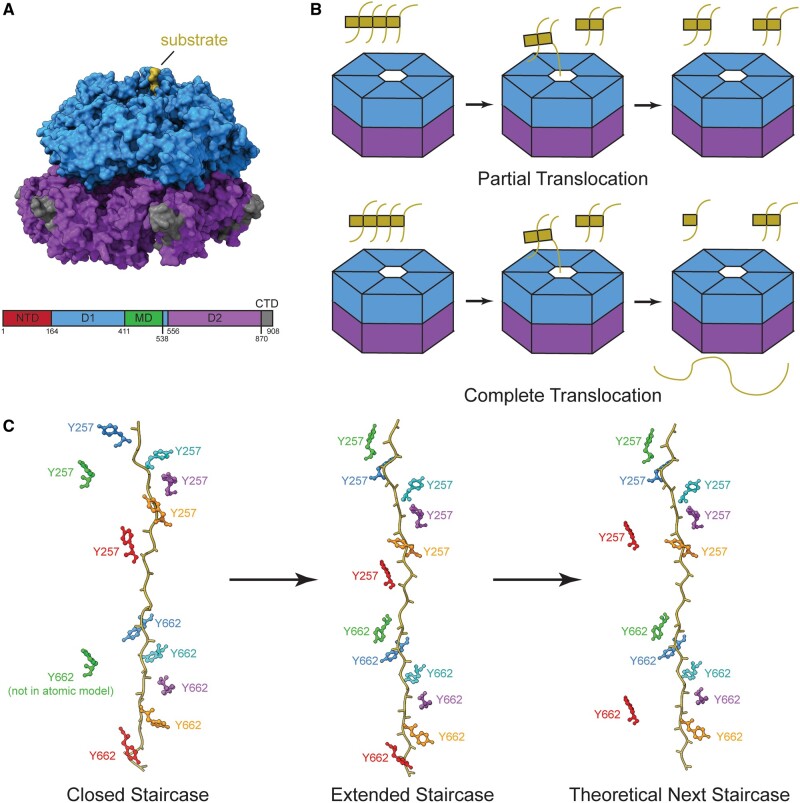
FIGURE 3.
The Hsp104 structure, different modes of translocation, and sequential processing of substrate using its pore loop staircase. (A) Upper: the surface view of Hsp104 ATPase domain 1 ring (D1, blue) on top of D2 ATPase ring (purple) bound to substrate (gold) (PDB: 5VJH). Lower: the domain architecture plot of Hsp104 including domains not in the structure shown above (N-terminal domain [NTD]: red, D1: blue, MD: green, D2: purple, CTD: dark gray). (B) Models of partial and complete modes of substrate translocation by Hsp104. Partial translocation does not pull substrate (gold) all the way through the pore, but releases it separated from other parts of the complex. Complete translocation unfolds substrate pulling it all the way through the Hsp104 pore, dissociating each monomer. (C) The possible order of events for Hsp104 staircase in substrate processing that shows a monomer binding to substrate (gold) at the top of the pore loop staircase prior to a monomer releasing substrate at the bottom of the pore loop staircase. The side chain colors correspond to each monomer and its movement through the staircase. Y257 is the key residue in pore loop 1 and Y662 is the key residue in pore loop 2 (PDB: 5JVH, 5VYA).