FIG 1.
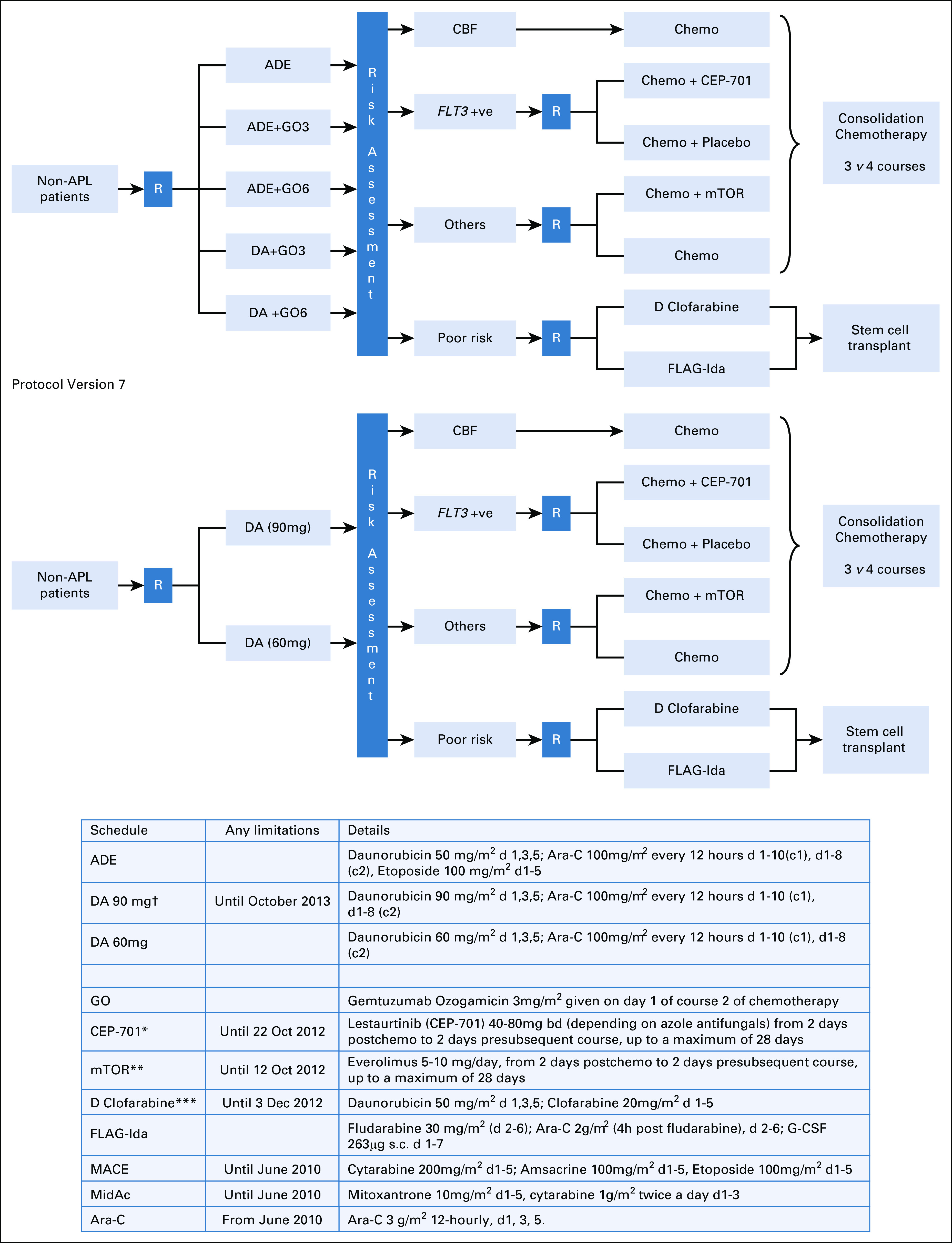
Protocol flow diagram. *Following closure of the CEP-701 randomly assigned, patients were guided by risk score to either poor risk or nonpoor risk options. **Following closure of the mTOR inhibition random assignment, patients in this group received DA 50mg alone. ***Following closure of the D Clofarabine arm, patients were recommended to receive FLAG-Ida (which was also the case if renal criteria were not met). †Following closure of the high-dose daunorubicin arm, patients were allocated DA60. ADE, Ara-C, daunorubicin, and etoposide; APL, acute promyelocytic leukemia; CBF, core binding factor; DA, daunorubicin and Ara-C; FLAG-Ida, fludarabine, Ara-C, granulocyte colony-stimulating factor, and idarubicin; GO, gemtuzumab ozogamicin; MACE, amsacrine, Ara-C, and etoposide; MidAc, mitoxantrone and Ara-C; mTor, mammalian target of Rapamicin.
