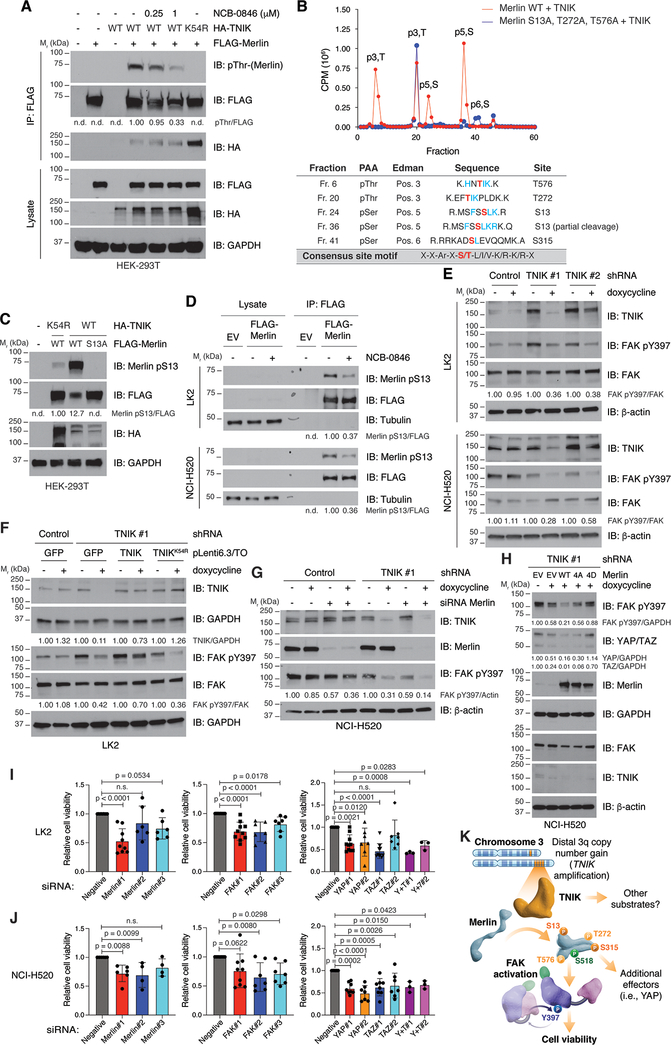
Figure 4. TNIK regulates Merlin and FAK in LSCC cells.
A. Overexpressed Merlin was immunoprecipitated from HEK 293T co-expressing TNIK or a kinase-dead (K54R) mutant. Merlin phosphorylation was assessed with a pan-phospho-threonine antibody, and interaction with TNIK was analyzed by immunoblot. Where indicated, cells were treated with the corresponding concentration of NCB-0846 for 30 minutes. The data are representative of three independent experiments. B. Purified Merlin was incubated with the kinase domain of TNIK in the presence of γ[32P]-ATP, and phosphopeptide mapping was conducted. The identified phosphorylated amino acids are indicated in red. PAA, phosphoamino acid analysis. The chromatogram is representative of three independent experiments. The TNIK phosphorylation consensus motif is shown in the bottom row. Conserved residues with the consensus motif around the identified phosphosites are indicated in blue. C. Validation of Merlin pS13 by co-expression of TNIK and Merlin wild-type (WT) or the non-phosphorylatable mutant S13A in HEK 293T cells from two independent experiments. D. Validation of Merlin pS13 in LK2 and NCI-H520 cells overexpressing Merlin and treated with NCB-0846 (500 nM, 90 minutes; n = 3 independent experiments for each cell line). E. Dox-inducible (1 μg/ml, 72 h) knockdown of TNIK reduces activation of FAK in LK2 and NCI-H520 cells. The data are representative of three independent experiments. F. Reduced activation of FAK in dox-inducible (1 μg/ml, 72 h) TNIK-knockdown cells can be rescued by expression of shRNA-resistant TNIK but not of a kinase-dead (K54R) mutant. The data are representative of three independent experiments. G. Combined knockdown of Merlin (96 h) and TNIK (with doxycycline 1 μg/ml, 72 h) results in FAK inhibition in NCI-H520 cells (n = 2 independent experiments). H. Reduced activation of FAK and YAP levels in dox-inducible (1 μg/ml, 72 h) TNIK-knockdown cells can be rescued by expression (48 h) of a Merlin phosphomimetic mutant (4D; S13D/T272D/S315D/T576D) but not by expression of Merlin wild-type (WT) or a non-phosphorylatable mutant (4A; S13A/T272A/S315A/T576A). The data are representative of three independent experiments. I, J. Effect of siRNA-mediated knockdown on LK2 (I) or NCI-H520 (J) cell viability (MTS assay). Data are represented as mean value ± SD, one-way ANOVA, Dunnett’s multiple comparison post-test (relative to siRNA negative). n ≥ 3 independent experiments with triplicates. n.d.: not detected. GAPDH, tubulin or β-actin were used as loading controls. Immunoblot band intensity was quantified with ImageJ. K. Proposed model on how amplified TNIK contributes to LSCC progression. In LSCC cells, TNIK phosphorylates Merlin at S13 and S315. In addition, TNIK might phosphorylate Merlin at secondary sites (T272 and T576). Phosphorylated Merlin is required to maintain activation of the focal adhesion kinase (FAK). Additional TNIK substrates or Merlin effectors (i.e. YAP) might also contribute to maintain LSCC cell viability.