Abstract
Extracellular vesicles (EVs) are a heterogeneous group of cell-derived membrane vesicles comprising apoptotic bodies, microvesicles and small EVs also called as exosomes. Exosomes when initially identified were considered as a waste product but the advancement in research techniques have provided insight into the important roles of exosomes in cell-cell communication, various biological process and disease, including cancer. As an important component of EVs, exosomes contain various biomolecules such as microRNAs (miRs), lipids and proteins that largely reflect the characteristics of their parent cells. Notably, cancer cells generate and secrete many more exosomes than normal cells. A growing body of evidence suggests that exosomes, as mediators of intercellular crosstalk, play a role in tumorigenesis, cancer cell invasion, angiogenesis, tumor microenvironment (TME) formation and cancer metastasis. As we gain more insights into the association between exosomes and cancer, the potential of exosomes for clinical use is becoming more intriguing. This review is focused on the role of exosomes in breast cancer, in terms of breast cancer biology, mechanism of action, potential as biomarkers and therapeutic opportunities.
Introduction
Breast cancer is the second leading cause of cancer death among women, and the most commonly diagnosed cancer among US women (1). Worldwide, more than two million women were newly diagnosed with breast cancer in 2018 (2).
The study of Extracellular Vesicles (EVs) has rapidly expanded in the past decade. Although the term exosomes is often used in general terms to describe vesicles isolated by different methods, we use it here to describe small EVs (sEVs) with the following characteristics: 1) originated from the endosomal pathway and release by multivesicular bodies (MVB) (3); 2) a size ranging from 30 to 150nm; and 3) a set of cell-derived cargos and surface markers specific for a given cell type (4). For decades, membrane protein markers such as tetraspanins (CD63, CD81, CD9) have been considered to be exosome-specific markers and have been widely used for exosome isolation and characterization. However, rapidly developing techniques and new knowledge constantly endow the name “exosomes” with new meaning. Using a combination of high-resolution density gradient fractionation and direct immunoaffinity capture (DIC), a recent study reported that there is a unique group of EVs, named non-classical exosomes, that share the same size and cellular origin with classic exosomes, but lack detectable tetraspanin markers (5). This indicates that EV may have an even more heterogenous composition than was originally appreciated. In the field of breast cancer, exosomes are being widely investigated for their application in cancer diagnostics, monitoring, prognostics and therapeutics. For example, some species of exosome-encapsulated miRs such as miR-1246, miR-21, are candidate biomarkers for early detection of breast cancer (6,7). Exosomes are also being explored as nanodrug delivery systems to deliver anti-miR (anti-microRNA) to alter cancer cell gene expression, or are loaded with anti-cancer drugs (8,9). At the same time, exosomes play a role in breast cancer invasion, metastasis, and tumor microenvironment (TME) formation (10,11). It is clear that our understanding of the role of exosomes in various processes such as cell-cell signaling is continually evolving and while many studies support that exosomes function to mediate intercellular interactions in the TME, the nature of these interactions require further investigation. Exactly how exosomes regulate cell-cell communication in the TME, who is directing the conversation between cell types and whether the exposure time (short-term or chronic) to exosomes could fundamentally define the course of the disease needs further exploration to determine the mechanistic aspects of issuing, transmitting, receiving and reacting to DNA, RNA and protein messages within exosomes.
Here we review current research specifically focusing on breast cancer cell communication mediated by exosomes, their interactions with stromal and immune cells, the potential clinical implications of exosome targeting and their use as disease biomarkers.
Exosome characterization
Exosomes arise from the endosomal pathway
Exosomes are small EVs originating from the endosomal pathway (Figure 1). Cells undergo endocytosis to form endocytic vesicles, which fuse together to produce an early endosome. Exosome formation begins with repeated invagination of the endosome lipid-bilayer, which generates intraluminal vesicles (ILVs) which are contained within the endosome called MVBs. These MVBs, which are sometimes called late endosomes, fuse with the plasma membrane to release exosomes outside of the cell. There are two independent mechanisms proposed for MVB formation and cargo sorting into the exosomes: 1) mediated via the endosomal sorting complex required for transport (ESCRT), and 2) via sphingolipid ceramide-mediated processes. The ESCRT machinery consists of four sequentially recruited complexes-ESCRT 0/I/II/III and other accessory components (12,13). Tsg101 and Alix, which have important roles in the assembly of the ESCRT machinery, are often used as markers to identify exosomes. For ESCRT-independent mechanisms, neutral sphingomyelinase and ceramide are required of exosome production. If not targeted for degradation by autophagy, the MVB will fuse with the plasma membrane, delivering exosomes into the extracellular space which relies on small GTPases such as RAb27a, Rab31 and Rab11 (12,13).
Figure 1. Classical model of the endocytic pathway, exosome biogenesis and release.
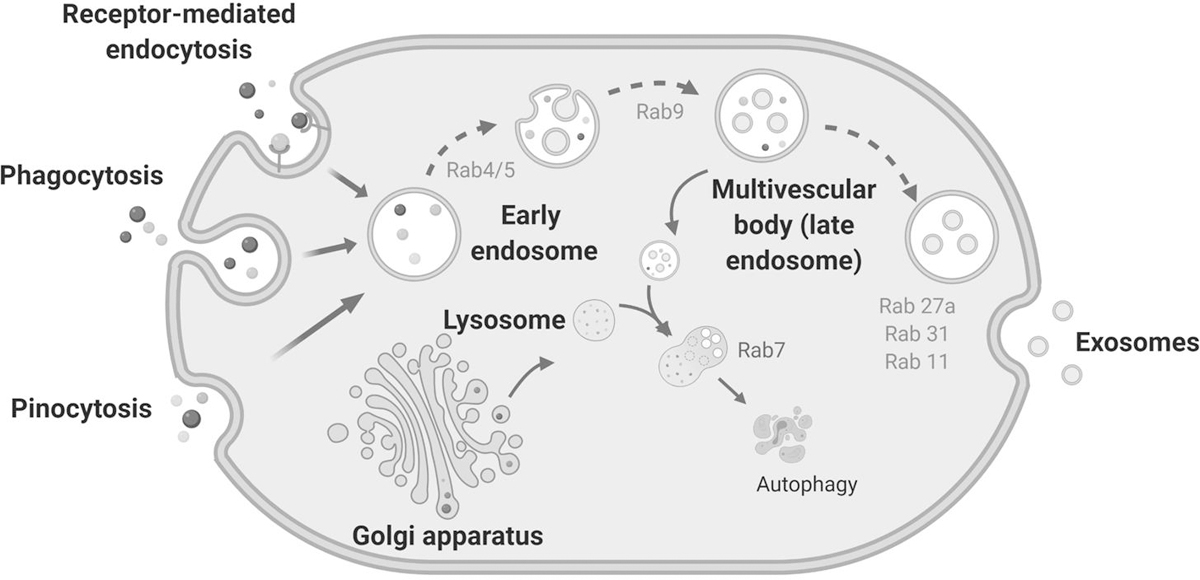
Exosome biogenesis occurs via the endosomal pathway. First, early endosomes are formed as a result of cell cytosis. During endosome maturation, the endosome membrane invaginates, giving rise to intraluminal vesicles (ILVs) which are packaged into multivesicular bodies or MVBs (also called late endosomes). The MVBs fuse with the plasma membrane and releases the ILVs, now termed exosomes, into the intracellular space. Alternatively, MVBs can fuse with lysosomes and are targeted for autophagy. The Rab family proteins play a critical role in the biogenesis of both multivesicular bodies and exosomes.
Exosomes carry a specific set of cell-derived cargo
A variety of cellular cargos are found inside exosomes, including nucleic acids (NAs), proteins and lipids (Figure 2). Well-known exosome-enriched proteins such as Tsg101 and those belonging to the tetraspanin family are frequently used as exosome markers in immunoblotting (5). In terms of DNA as a cargo, it is reported that more than 90% of amplifiable plasma circulating-free DNA (cfDNA) is associated with plasma exosomes, including single-stranded DNA and possibly double-stranded DNA, a large fraction of which is ctDNA in cancer patients (5,14,15). DNA-seq and RNA-seq of exosome derived NAs revealed that exosomal DNA (exo-DNA) and exosomal mRNA (exo-mRNA) are an unbiased representation of the human genome and transcriptome, while some species of microRNA are greatly enriched in exosomes compared to cells, along with other species of non-coding RNA (16). Exosomal microRNAs are a major form of extracellular microRNAs. The sorting and packaging mechanisms for exosomal microRNAs are previously well summarized (17,18). In cancer cells, microRNAs are frequently deregulated, packaged in tumor associated exosome (TAEs) and actively secreted into bodily fluids, making them ideal biomarkers for cancer (19). Exosomal microRNAs as biomarkers in breast cancer will be discussed further in this review. Furthermore, recent studies have demonstrated that the exosomal proteome can be used to distinguish between tumor and non-tumor tissues (20), this highlights the application of exosomes in early detection of cancer and to classify primary tumor types.
Figure 2. Exosome structure and composition.
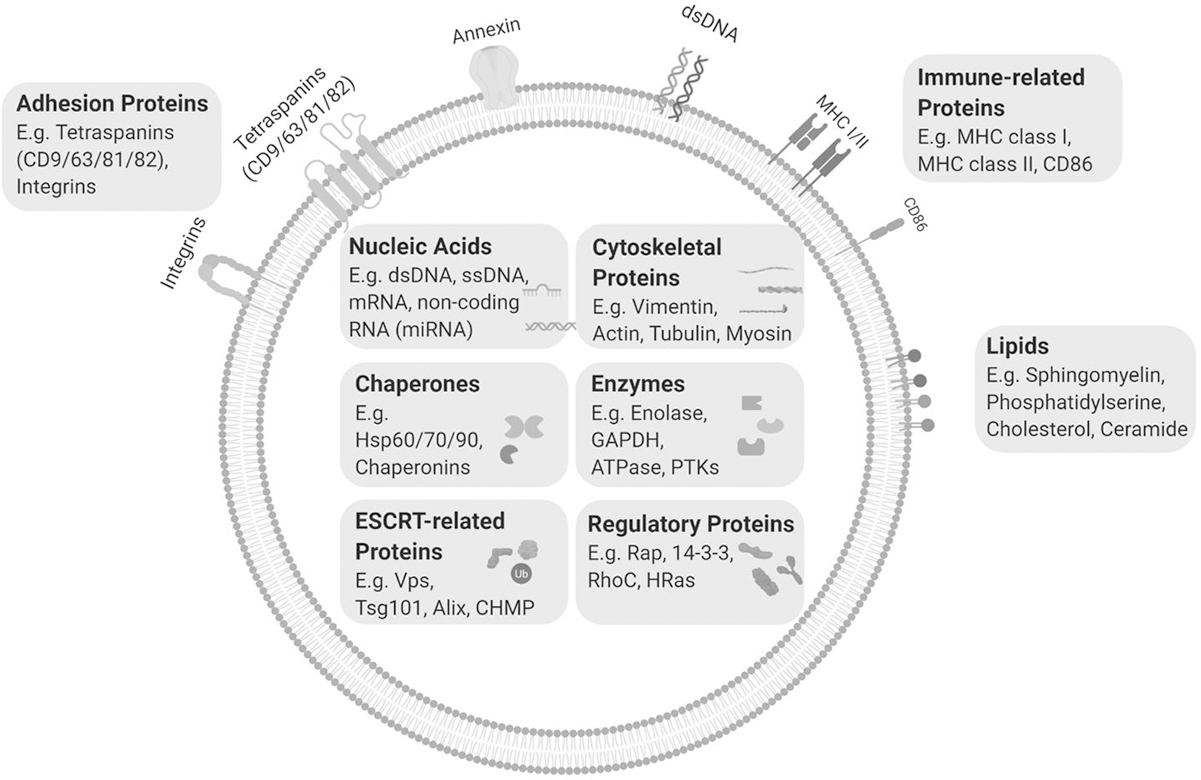
Exosomes have a heterogeneous composition and can host a wide array of cargos including DNA, RNA, microRNAs, proteins and lipids. Individual exosomes contain a specific set of proteins and RNAs that characterize their parent cells and pathological state and account for their biological functions. Exosome cargo can exist inside the lumen of the exosome or within its lipid bilayer. MHC I/II, Major histocompatibility complex I/II; HSP60/70/90, heat shock protein 60/70/90; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; PTK, protein kinase; Vps, vacuolar protein sorting-associated protein; CHMP, charged multivesicular body protein; Rap, Ras-related protein; RhoC, Rho-related GTP-binding protein.
Exosomes can be isolated and characterized using multiple methods
Exosomes are released by both normal and cancer cells. They are found in almost all types of body fluids including blood (21) (Figure 3). When isolated, exosomes are not easily degraded by repeated freezing and thawing, protecting the entities inside, which greatly strengthens their utility as biomarkers.
Figure 3. Exosome isolation and characterization: A Key step for the study of exosome content and function.
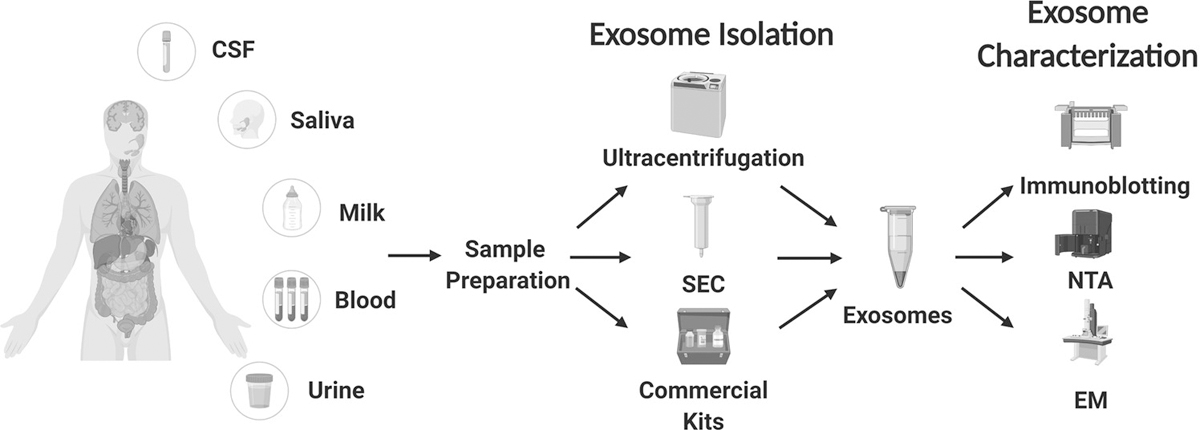
Exosomes are found in many types of body fluid and can be isolated by a variety of methods such as ultracentrifugation, size-exclusion columns (SEC) and marker based pull-down assays (commercial kits). Isolated exosomes are characterized by a combination of techniques including staining for exosome-specific protein markers (using immunoblotting), nanoparticle tracking analysis (NTA), and electron microscopy (EM). These techniques have been widely applied to evaluate the quality and concentration of isolated exosomes. Of note, the methods for exosome isolation, purification and characterization are developing rapidly as insights are gained into the biological features of exosomes. CSF, cerebrospinal fluid.
Using a high-resolution intravital real-time imaging system, Anoek et al. were able to visualize exosome trafficking among breast cancer cells and stromal cells in mice (22). More generally, a wide variety of methods have been developed and compared to isolate exosomes from cell culture media or body fluids to characterize them in vitro (23). Centrifugation-based methods have been widely used and compared (24). These methods require the pre-clearing of cells and cell debris from blood or cell culture media by multiple steps of centrifugation, then exosomes are pelleted and recovered during ultracentrifugation (100,000x). Though inexpensive and scalable, the method cannot efficiently separate large protein molecules and exosomes (25). Exosomes of the highest purity can be acquired by density gradient separation, though this usually requires a large amount of starting material (26). Size-exclusion chromatography (SEC) enriches exosomes by vesicle size, efficiently removing contamination caused by large proteins or protein aggregates and is widely used for exosome isolation from serum or plasma (27), despite low exosome recovery rates.
To confirm the successful isolation of exosomes, the joint use of complementary techniques is proposed. Immunoblotting, Electron microscopy (EM) and Nanoparticle tracking analysis (NTA) are commonly used for exosome characterization (28). EM is valuable for visualizing the microstructure and size of exosomes, and determines the vesicle heterogeneity while NTA or resistive pulse sensing can efficiently measures the concentration and size distribution of vesicles (29–31). Immunoblotting for positive and negative markers of exosomes validates successful isolation and rules out the possibility of cellular protein contamination (4). In addition to these classical methods, novel techniques such as Raman tweezers microspectroscopy (RTM) and flow cytometric methods have been proposed to characterize the global chemical composition or surface markers of exosomes (28,32). Currently much of the literature on exosomes, including those studies cited here, are limited in their characterization of the EVs they are studying, and the field needs to standardize and validate isolation methods and agree on nomenclature in order to draw stronger conclusions from the available research covering a broad spectrum of vesicle types due to the molecular and functional heterogeneity of EVs.
Exosomes in breast cancer: from tumor macroenvironment to tumor microenvironment
Metastatic cancer poses the biggest threat to all cancer patients and is responsible for > 90% of cancer-related deaths (33). According to the original “seed and soil” theory by Stephen Paget, the “seed”-cancer cells may spread but only form metastases in congenial “soil” (34). During the process of metastatic cancer development, signals produced by tumor cells at the primary site are sent to other locations of the body, fertilizing the “soil” and establishing the pre-metastatic niche, making it suitable for circulating tumor cells to seed thereafter. In other words, a host-tumor “macroenvironment” is formed (35). Breast cancer is not a single disease, but a heterogeneous group of diseases possessing both inter- and intra-tumor heterogeneity. The histopathologic heterogeneity in breast cancer can be illustrated based on the physical exam and imaging. Accordingly, the morphological features can classify breast cancer into different subtypes, of which IDC (invasive ductal carcinoma) and ILC (invasive lobular carcinoma) are the major subtypes accounting for 40–75% and 5–15% of the total cases, respectively. Breast cancer can be subclassified based upon the expression of ER (estrogen receptor), PR (progesterone receptor) and HER-2 (human epidermal growth receptor 2) proteins. Further, molecular heterogeneity classifies the disease into five major subtypes based on the expression of gene signatures including Luminal A, Luminal B, Triple-negative/basal-like, HER2-enriched, and normal-like. The biological behavior of BCCs in different subtypes has proved substantially different. Accordingly, the metastatic pattern of breast cancer subtypes varies and has been stressed as “organotropism”, which means that a certain type of BCC displays an obvious preference to metastasize to one or a few specific organs. For example, TNBC has been shown to have higher rates of metastases to brain, lung and liver. The phenomenon of organotropism is complex and is shown to be regulated by the subtype of breast cancer, the host microenvironment of distal organs, the interaction between disseminated cancer cells (DTCs) and local cells, and secretory and receptive traits of BCCs. For example, Chen et al. showed that TNBC cells release exosomes that carry immunosuppressive PD-L1 protein in vitro, and Yang et al. demonstrated that these exosomes attenuate cellular immunity against BCCs and contribute to tumor growth in vivo (36,37). On the contrary, exosomes secreted by PD-L1-low MCF7 (positive for hormone receptors) do not have similar effect (37). Of note, a comprehensive analysis and comparison of exosomal proteome in various subtypes of BCC is further warranted to better characterize BCC secretome, intercellular crosstalk during the establishment of pre-metastatic niche, and the organotropic metastasis of breast cancer.
Exosome-mediated cell-cell signaling can occur during different phases of breast cancer metastasis and plays an important role to modulate the host microenvironment to establish a pre-metastatic niche. In this context, it has been shown that exosomal miR-105 can efficiently disrupt cell junctions of endothelial cells which accelerates the intravasation of localized BCCs (38). Furthermore, It has been shown that the exosomes secreted by tropic tumor cells can be uptaken preferentially by organ cells of respective destination (10), indicating that stromal cells can differential uptake tropic tumor cell exosomes in metastatic target organs. The bidirectional communication between BCCs and stromal cells continues when micrometastatic or metastatic tumor has formed, which educates cells of both sides and facilitate TME formation (39,40). This is how cell-particle-cell communication multidimensionally promotes breast cancer metastasis.
The role of exosomes in intercellular communication between breast cancer cells (BCCs) and cells in the TME is a research hotspot because secreted substances are key components of TME (Figure 4). For example, the injection of exosomes from BCCs with metastatic tropism to a specific organ was shown to facilitate or redirect the metastatic behavior of BCCs with a different organ preference or without organotropism (10,41). This indicates that tumor exosomes may help to establish a premetastatic niche and mediate organ tropism. Rodrigues et al. showed that the type of integrins on the tumor exosome membrane, which may ligate with ligands at distant organs, may determine exosome uptake by organ-specific cells and thus affecting the organ tropism of breast cancer metastasis (10). They further showed that integrins α6β1 and α6β4 were associated with lung metastasis while αvβ5 was linked to liver metastasis (10). The same group also reported that the uptake of tumor exosomal CEMIP protein by brain endothelial and microglial cells promotes vascular remodeling and inflammation, leading to the formation of a pre-metastatic niche (42). These studies provide enough evidence to show that exosomes play a key role in establishing a pre-metastatic niche by modulating the host tumor microenvironment to support breast cancer metastasis and organotropism.
Figure 4. Exosomes mediate cell to cell crosstalk in the TME.
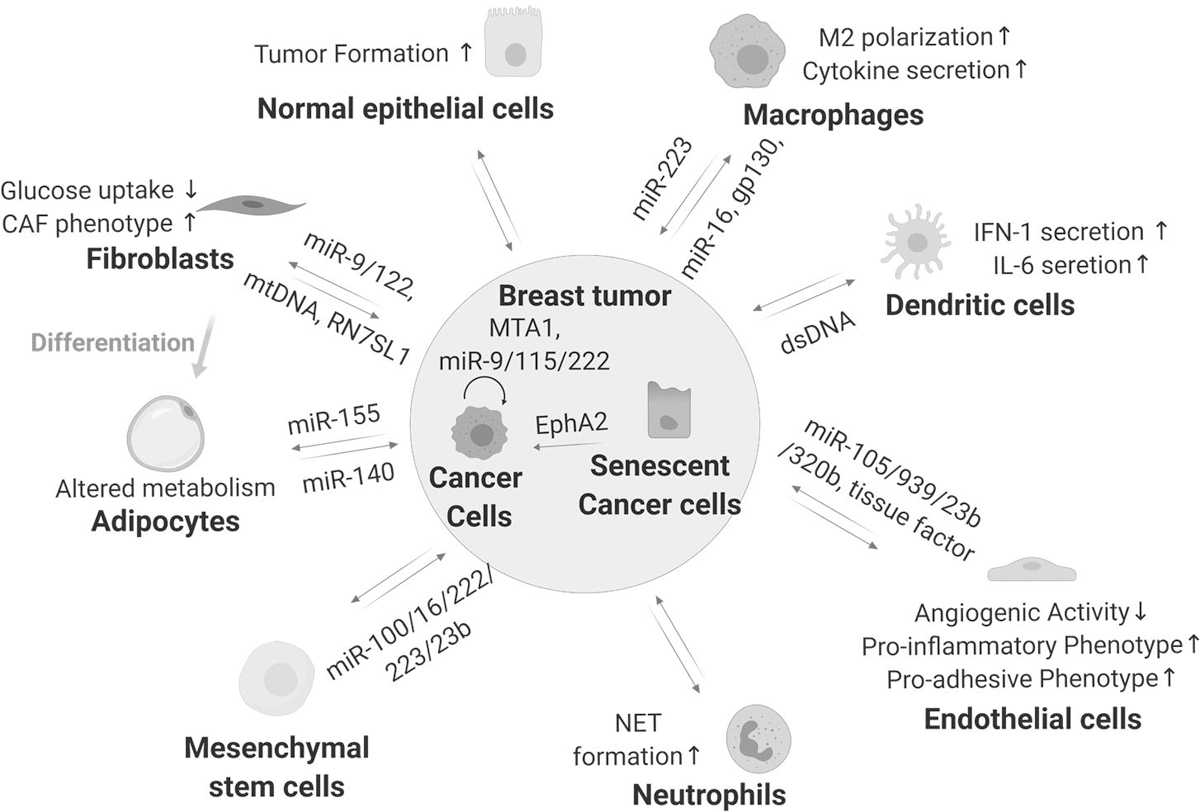
Cellular components of the tumor microenvironment (TME) includes malignant cells such as breast cancer cells (BCC) and non-malignant stromal cells such as immune cells, mesenchymal stem cell (MSC)-derived cell populations, endothelial cells and epithelial cells. Exosomes released by BCCs may transform the function of cells in the TME to favor breast cancer progression and metastasis. Studies also suggest that BCC derived exosomes can mobilize neutrophils and skew M2 polarization to promote breast cancer progression as well as activating endothelial cells to support tumor angiogenesis. BCCs can also uptake exosomes from the TME. This bidirectional exosome-mediated transfer of cellular substances, such as proteins and nucleic acids, greatly affects the dynamics of the TME and likely contributes to breast cancer progression and metastasis.
Even after the cancer cells find a congenial soil, they usually “hide” in the form of micrometastases rather than expanding immediately. After many years, small aggregates of cells exit dormancy and grow into clinically significant macrometastases (43). Cancer cells are not completely inactive during dormancy. They recruit and educate stromal cells and immune cells into tumor supportive cells such as cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs), which contribute to the TME, a tumor-favoring niche in which the tumor cells survive or progress, and which includes blood vessels, tumor associated cells, and the extracellular matrix (ECM) (44). The establishment of the TME relies largely on cellular complexity and dynamic intercellular signaling between cancer cells and non-transformed supporting cells (45) and exosomes play an important role in this intercellular signaling/communication. These signals include traditional cytokines, chemokines, growth factors, inflammatory mediators and matrix remodeling enzymes, and reactive stromal cells co-opted by the tumor, go through further transition into tumor-associated stromal cells (TASCs) (46). Not only are tumor cell-derived exosomes part of the intercellular communication to establish the tumor macroenvironment, but they are crucial to formation of TME. This vicious BCC-stromal cell crosstalk, in part facilitated by exosomes, continuously contributes to the accumulation of tumor mass.
The overall relationship between exosomes, cancer metastasis and TME has been extensively reviewed, previously (47–49). This article focuses on the current evidence highlighting the role of exosomes in cell-cell communication between different cell types in the breast cancer disease. It should be noted that the majority of the studies discussed here use a combination of in vitro exosome isolation methods coupled with in vitro or in vivo phenotypic studies. This approach provides compelling data for the role of exosomes in cell-cell communication, with direct in vivo evidence in several studies, though extensive studies are still required to truly demonstrate that what is being studied are exosomes compared to other types of EVs, the origin of these exosomes, their cargo and their ultimate targets in recipient cells.
Exosome-mediated communication among BCCs and with non-transformed epithelial cells
Cell-to-cell crosstalk between subpopulations within the TME may be accomplished by exosome secretion. A variety of studies have been reported that assess the effect of exosomes derived from non-transformed epithelial cells on cancer cell phenotypes, as well as exosomes exchanged between tumor cell populations. Almost two-thirds of breast cancer cases are estrogen receptor (ER) alpha positive and estrogen signaling plays an important role in BCC viability and growth. Recent studies have shown that exosomes may play a critical role in regulating estrogen signaling in breast cancer. Using in vitro BCC models, it has been shown that BCC-derived exosomes containing the transcriptional co-regulator MTA1 may regulate estrogen signaling and hypoxic response in recipient cells in vitro, suggesting a role for exosomes in promoting communication between breast cancer subpopulations (50). MicroRNAs contribute to diverse cellular processes and are a key exosomal cargo, which may modulate the behavior of recipient cells. Studies have shown that metastatic BCC derived exosomes that are enriched in miR-9 and miR-155 can be taken in by recipient BCCs and decrease the expression of the tumor suppressor genes PTEN and DUSP14, in turn promoting the tumorigenicity of the recipient cells (51). Similarly, Ding et al. demonstrated that BCC cells with low metastatic ability exhibited enhanced migration and invasion after receiving exosomes containing miR-222 at least in part due to the subsequent down-regulation of the PDLIM2 tumor suppressor gene and activation of the NF-kB pathway (52). Further, miR-222 was increased in breast cancer patient plasma derived exosomes, suggesting a potential clinical relevance to their findings (52). While mounting evidence supports a key role for exosomal miRs in cell-cell communication, each miR has many cellular targets making understanding their effects in target cells highly complex. Therefore, extensive validation studies are required to demonstrate the effect of tumor or stromal derived exosomal miRs in target responder cells in order to determine the mechanism of their function.
Breast cancer cells arise from transformed epithelial cells in breast tissue. During and after transformation, cancer cells continue to communicate with surrounding normal epithelial cells, sending or receiving various signals of growth or survival (53). Several recent studies provide evidence that exosomes may play a critical role to facilitate this bidirectional communication. Melo et al. showed BCC exosomes can efficiently promote normal breast epithelial cells to form tumors in vivo (54). Meanwhile, a study by Dutta et al. reported that exosomes from breast cancer cell lines can induce reactive oxygen species (ROS) and autophagy in human primary mammary epithelial cells (HMECs) followed by an induction of the DNA damage response (DDR) and p53 stabilization (55). Conversely, epithelial cell derived exosomes may inhibit exosome secretion by BCCs in a tissue-specific manner (56). A study by Takasugi and colleagues showed that in senescent non-malignant epithelial cells, packaging of EphA2 into exosomes is increased and that these exosomes may enhance the proliferation of MCF7 cells in vitro, suggesting that non-transformed epithelial cells can support the growth of tumor cells in the TME through exosomes (57). While these studies provide compelling evidence to support the role of exosomes between BCCs and non-transformed epithelial cells, further in vivo studies are warranted to decipher the underlying mechanisms of signaling and the specificity of exosome mediated signaling.
Exosome-mediated communication between breast cancer and immune cells
The role of immune cells in tumorigenesis is a double-edged sword. On one hand, immune cells help to enhance the anti-tumor immune response and act to eliminate cancer cells; while on the other, immune cells can be corrupted by cancer cells and become pro-tumorigenic. Interestingly, exosome signaling has been shown to play a pivotal role in the crosstalk between immune and cancer cells (58). Here we summarize exosome-mediated interactions between BCC and dendritic cells (DCs), macrophages, natural killer cells, cytotoxic T lymphocytes, and other specialized immune cell types.
Cytokines are inducible molecules which play an important role in intercellular communication between immune cells, inflammation, tumor initiation and tumor metastasis. Interestingly, it has been shown that breast cancer exosomes can activate cytokine production. For example, Maji et al. showed in vivo that MDA-MB-231 released exosomes are taken up by macrophages which is associated with higher secretion of IL1, IL6, and TNFα (41). In addition, M2 macrophages are known to have anti-inflammatory and pro-tumorigenic effects (59) and exosomes are shown to modulate the polarization of macrophages. Ham et al. reported that BCCs exosomes transfer gp130 to macrophages in vitro, which alters macrophage polarization by activating the STAT3 pathway (60). In vitro and ex vivo studies suggest that epigallocatechin gallate treated 4T1 cells secrete exosomes that contain miR-16, which can then be transferred to macrophages and enhances M2 polarization (61). On the contrary, BCCs may be influenced by exosomes secreted by macrophages, such as activated tumor-associated macrophages (TAM) which may promote BCC invasion by exosomal transfer of miR-223 (62). Using an in vivo BCC dormancy model, Walker et. al reported that M1 macrophage exosomes can reverse BCC quiescence and induce BCC proliferation (63). This sheds new light on the role of M1 macrophages in breast cancer mediated by exosomes.
DCs are professional antigen-presenting cells that respond to tumor-associated antigens. When treated with the topoisomerase inhibitor Topotecan or irradiated, BCCs release exosomes containing immunostimulatory dsDNA, which activates STING signaling in DCs and induces IL6 production and IFN-1 activation. (64,65). Ning et al. recently demonstrated that exosomes from 4T1 cells caused apoptosis of DCs and suppressed their maturation (66), highlighting one more process that could be modulated by exosomes.
Lastly, neutrophils can also communicate with BCCs through transfer of exosomes. Under normal circumstances, neutrophils protect against microbial infections and are facilitators of wound healing. However, in the TME, they are manipulated by cancer cells to create a favorable state for tumor growth (67). In a study by Leal et al., BCC-derived exosomes induced the formation of neutrophil extracellular traps (NET), promoting cancer associated thrombosis (68). It is likely that different exosome cargoes are responsible for the differential effects on immune cells and in regulating cellular crosstalk. While further studies are needed there is a growing body of evidence supporting cancer and immune cell crosstalk via exosomes which may have clinical importance.
Exosome-mediated communication between BCCs and stromal fibroblasts
Exosome-mediated intercellular signaling between cancer cells and stromal fibroblasts can regulate breast cancer progression, drug resistance, and cancer promoting inflammatory responses. RNA transferred by stromal fibroblast exosomes can stimulate both STAT1 signaling in BCCs and pattern recognition receptor (RIG1) on immune cells, which together promote the proliferation of therapy-resistant BCCs (39). Specifically, Nabet et al. reported that fibroblast released exosomes containing RNA RN7SL1 can be delivered to BCCs to enhance tumor metastasis and therapy resistance (69). This adds to the understanding of how damage associated molecular patterns (DAMPs) promote aggressive features of breast cancer. Using patient-derived xenografts (PDXs), Pasquale et al. demonstrated that exosomes can carry mitochondrial DNA (mtDNA) from (mouse) fibroblasts to (human) BCCs, leading to enhanced oxidative phosphorylation and escape of BCC from dormancy and increased therapy resistance (70). However, further studies are required to understand mtDNA transfer via exosomes and its relevance in BCC development and progression. In addition to exosomal NAs, exosomal proteins also have a role in cell-cell crosstalk. Luga et al. exploited both in vivo and in vitro breast cancer models to demonstrate that fibroblast-derived exosomes promote BCC protrusive activity and migration by activating the Wnt-regulated planar cell polarity pathway and these phenotypes were inhibited following knockdown of CD81 (71).
At the same time, stromal fibroblasts can also receive signals carried by exosomes from BCCs. CAFs are involved in tumorigenesis and studies demonstrate that normal fibroblasts exhibit CAF phenotypes after uptake of exosomal miR-9 from BCCs, in turn promoting in vivo tumor growth (72). Similarly, BCC exosomes containing miR-122 decrease glucose uptake of lung fibroblasts, facilitating establishment of a premetastatic niche with reprogrammed glucose metabolism. Thus a tumor-favorable microenvironment may accommodate the increased energy consumption of BCCs during growth and metastasis (73).
A subgroup of fibroblasts transdifferentiate into adipocytes (74). Cancer associated adipocytes (CAAs) are adipocytes that are reprogrammed to an activated state with phenotypic changes during interaction with tumor cells. CAAs generally exist at the invasive front of tumors, showing enhanced ECM-remodeling and cancer-promoting ability compared to their normal counterparts (75). In the TME, adipocyte precursor cells, also named preadipocytes, play a role in tumor-stomal interaction (76). Gernapudi et. al reported that after treatment of preadipocyte-derived exosomes which contain miR-140, ductal carcinoma in situ (DCIS) cells showed enhanced stem cell activity and self-renewal ability in vitro, and tumor formation in vivo (76). Conversely, BCC exosomes containing miR-155 can reprogram the metabolism of adipocytes, which may promote metastasis (11). By and large, these studies highlight the importance of exosome mediated crosstalk between BCCs and fibroblasts and suggest an important role in tumor progression and development which is just beginning to be understood.
Exosome-mediated communication between BCCs and mesenchymal stem cell
Mesenchymal stem cell (MSCs) are a group of multipotent stem cells with the potential to differentiate into a variety of mesodermal lineages including chondrocytes, osteoblasts, adipocytes, pericytes, muscle cells and fibroblasts. Stromal fibroblasts, pericytes and adipocytes are key components of tumor stromal cell population (45). Evidence suggests that exosome-mediated cell-cell interaction between BCCs and MSCs contribute to TME establishment (77,78). Using breast cancer cell models, Pakravan et al. showed that MSC exosomes lead to a significant decrease of vascular endothelial growth factor (VEGF) secretion by BCCs. The authors showed that this is due to exosomal miR-100 and miR-16 modulation of the mTOR/HIF-1α signaling axis in BCCs (79). As with other cell types, the crosstalk mediated by exosomes is bidirectional and studies show that after pre-exposure to BCCs, MSCs may produce exosomes which can in turn promote BCC proliferation, establishing a feed-forward communication loop. Conversely, exosomal miR222/223 from MSCs may keep BCCs from escaping quiescence and confer drug resistance (40). Similarly, Ono et at. also demonstrated that bone marrow MSCs promote BCC dormancy by transferring miR-23b (80). Although the studies are limited, researches focusing on exosome-mediated MSC-BCC interactions will likely further study these avenues with the goal of exploiting them for the prevention and management of cancer relapse.
Exosome-mediated communication between BCCs and endothelial cells
Lining the lumen of blood vessels, endothelial cell plays a critical role in neo-angiogenesis. In the context of cancer, excessive proliferation of endothelial cells are usually associated with cancer development and progression (81). The communication between cancer cells and endothelial cells is important in three ways. First, increased tumor mass requires increased blood flow and nutrient delivery to maintain growth, during which the expansion of blood vessels is vital. Second, tumor cells must break into vessels to enter the bloodstream (intravasation) and then exit the blood stream (extravasation) in order to colonize distant sites (81). Third, the secretory function of endothelial cells are required to establish a microenvironment (82). Multiple studies show that exosome-mediated cell-to-cell signaling between endothelial cells and BCCs is crucial to breast cancer progression.
Zhou et al. provided evidence that exosomes from metastatic BCCs induce the migration of endothelial cells in vitro, via the targeted degradation of tight junction protein ZO-1 by exosomal miR-105 (38). Using mouse models, they further demonstrated that the injection of miR-105-high exosomes lead to increased vascular permeability at multiple sites. Furthermore, the mice developed multi-organ metastases after injection of miR-105-overexpressing BCCs, in contrast to the control group (38). BCC exosomal miR-939 can also reprogram endothelial cells, altering their barrier functions by downregulating VE-cadherin (83). BCC exosomes are also associated with angiogenic activity and quiescence of endothelial cells. When treated with the anti-angiogenic Docosahexaenoic acid (DHA), the microRNA contents of BCC exosomes are altered, and increased miR-23b/320b reduced endothelial cell vessel formation in vitro (84). Further, BCC exosomes containing tissue factor can confer a pro-inflammatory and pro-adhesive phenotype to endothelial cells in a PAR-dependent manner (85). Of note, the majority of the studies discussed here explored the role of exosomal miRNAs in the cross talk between BCCs and other cell types. This suggests that the role of exosomal proteome is yet to be explored further in this cross talk.
While there is increasing evidence for the significant role of exosomes in breast cancer, many questions remain. For example, it is yet to be determined how exosomes play a part in BCC intravasation and extravasation, or how exosome regulation of angiogenesis differs between primary breast tumors and distant metastasis. As with other cell types, further research is warranted to understand the complex relationship between BCC and endothelial cells and its role in cancer progression.
Exosomes and clinical application
Exosomal biomolecules as breast cancer biomarkers
Efficient screening for breast cancer allowing early diagnosis and monitoring of disease is critical for improved outcomes. To this end, multiple biomarkers including tissue markers (for example hormone receptors, HER2, Ki67), genetic markers (such as BRCA1/2) and serum markers (CA 15.3, CA549) have been developed (86). Many of them, however, either require an invasive tumor biopsy, or cannot provide comprehensive information about the cancer status, making “precision medicine” challenging to achieve. Tumor-derived exosomes, as an important part of liquid biopsies (i.e. sampling and analysis of non-solid biological tissue, largely blood), are promising biomarker candidates due to their tumor cell origin, stability, heterogeneous constituents (protein, DNA, RNA, lipid, etc.), and easy-to-obtain nature. For example, tumor exo-DNA can reveal the mutational status of cancer cells (87). Given that every tumor has a unique genetic footprint, exosomes provide a unique opportunity for enabling precision medicine guided detection and treatment of breast cancer.
Blood serum miRNAs are biomarkers of breast cancer and as this review has highlighted, miRNAs are enriched in exosomes, though understanding their function in target cells requires further characterization (88). Using small RNA sequencing, Hannafon et al. reported that exosomal miRs, miR-1246 and miR-21, are significantly elevated in breast cancer patient plasma, and may indicate the presence of breast cancer (7). In addition, exosomal miRNAs may be biomarkers of treatment response. Wu et al. found that plasma derived exosomal miR-375 and miR-122 can predict neoadjuvant response and breast cancer recurrence in stage II and III breast cancer patients (89). In a randomized phase II neoadjuvant clinical trial (GeparSixto, NCT01426880) which recruited patients with HER2-positive breast cancer and triple-negative breast cancer (TNBC), an exosomal miRNA signature was able to predict multiple clinical parameters such as pathological complete response (90). In the same trial, plasma exosomal miR-374 was associated with tumor size in patients with TNBC, while another group of exosomal miRNAs (miR-382, miR-185, miR-376a, miR-410, miR-628, miR-433) could be used in the same way for patients with HER2-positive disease (90).
These studies suggest a role of exosomal miRNAs derived from patient blood samples as potential clinical biomarkers of breast cancer diagnosis and treatment response however, the majority of studies lack direct evidence demonstrating origin of the exosomes and target engagement in responder cells. Although exciting, exosome miRNA studies need to be interpreted with caution until in-depth studies are available demonstrating the origin of these miRNAs (tumor or other cell types), their targets in responder cells and the biological association with breast cancer progression.
A number of cancer-related proteins are present in tumor exosomes. Using a pluronic block-copolymer based enrichment method followed by proteomic profiling, Zhong et at. showed that 60 exosomal proteins, including epidermal growth factor receptor (EGFR), could separate advanced breast cancer patients from a normal control group (91). Similar to exosomal miRNAs, some exosomal proteins can potentially predict therapy resistance. In a cohort of breast cancer patients, Teng et al. showed that in unresectable metastatic breast tumors, increased blood exosomal TRPC5 was related to chemotherapy response and acquired chemoresistance (92). Similarly, exosomal GSTP1 can be upregulated in chemoresistant breast cancer tissues and patient blood (93). Finally, serum exosomal CD82 levels was able to distinguish patients with breast cancer and healthy subjects (94).
Although the clinical application of exosomes in cancer diagnosis and monitoring is intriguing, this research area is still in its infancy and additional studies are needed to understand the diagnostic and prognostic utility of exosomes and to standardize procedures and analyses for clinical applications.
Exploiting exosomes as drug delivery systems
As we discussed in the previous sections, exosomes are natural carriers of various biomolecules to fulfill cell-cell communication. In the era of precision medicine and individualized therapy, these nanovesicles have the potential for specific drug delivery due to their non-immunogenic, targetable and degradable nature, maintaining an advantage over synthetic drug carriers (95). Studies have shown that exosomes can deliver therapeutic proteins and RNAs directly into target cells (96,97). A study by Jung et al. suggested that under hypoxic conditions, MDA-MB-231 can secret exosomes that are preferentially taken in by hypoxic cells in vitro (98). Injection of Olaparib-loaded exosomes intratumorally resulted in cancer cell apoptosis and delay of tumor growth (98). Exosomes secreted from stromal cells can have similar functions, for example, mesenchymal stem cell-derived exosomes were able to deliver paclitaxel (PTX) and miR (mirNa-142–3p) inhibitor into mouse breast tumors and inhibit in vivo tumorigenesis (8,9). The targeted delivery of anti-cancer drug remains a challenge in cancer therapy, and the modification of exosome surface proteins might enable such a targeting approach. By expressing a fusion protein which contained GE11 oligopeptide in donor cells and introducing miRNA into GE11-positve exosomes, Ohno et al. were able to deliver let-7a, a putative tumor suppressor, to EGFR-positive breast cancer cells in vivo to inhibit tumor growth (99). Similarly, by conjugating Designed Ankyrin Repeated Proteins to the exosome-specific protein LAMP2, Gomari et al. were able to generate exosomes that specifically recognize HER2 on BCCs, and increase the efficiency of drug delivery (100). Vesicle-based drug-delivery are promising to overcome limitations of conventional anti-cancer drug administration, while bioactive exosomes have the potential to improve breast cancer therapeutics.
Disruption of exosomes as a novel strategy to treat breast cancer
Due to the important role of exosomes in intercellular communication and the establishment of the TME, disruption of this communication may be a novel way of managing breast cancer. Different steps including exosome release, exosomal transfer of biomolecules, and exosome uptake are potential drug targets. Release of exosomes has been shown to affect drug retention in cancer cells in vitro whereby blocking exosome secretion by small molecules can increase the sensitivity of cells to chemotherapeutic agents such as 5-fluorouracil (101). In addition to data suggesting that blocking exosome secretion may alter response to chemotherapies, recent immunology studies suggest that exosomes could be potential mediators of response to immunotherapy. For example, PD-L1 is trafficked via exosomes involving a protein ALIX. When ALIX is depleted, PD-L1 undergoes reduced exosomal trafficking and PD-L1 levels increase on the cell surface. Consistent with this, ALIX depleted cells show enhanced immunosuppression of T cells (102). Taken together, these studies open new avenues to explore exosomes as targets of novel therapeutic strategies.
In addition, Nishida-Aoki et al. demonstrated that antibodies targeting CD9 and CD63 can stimulate the active removal of cancer-derived exosomes by macrophages (103), which could be a novel cancer therapy. They showed in vivo that when mice bearing MDA-MB-231 xenografts were treated with human anti-CD9 and anti-CD63 antibodies, metastases were significantly reduced (103) suggesting that exosome depletion is an approach worth further attention, however, it remains a challenge to distinguish and selectively deplete only cancer-derived exosomes and not other beneficial exosomes simply based on surface markers. To address this, validated markers or techniques are necessary which can discriminate subpopulations of exosomes effectively. Overall, these limited yet interesting studies show that exosomes hold potential as being novel drug targets.
Exosomes in breast cancer clinical trials
Clinically, exosomes are being evaluated as diagnostic tools and as biomarkers predictive of treatment response. There are on-going clinical trials focusing on exosomes as cancer biomarkers in patients with breast cancer. For example, one trial (NCT02977468) is determining whether exosomes can serve as biomarkers of response to pembrolizumab in patients with TNBC. In another clinical trial (NCT02662621) exosomal HSP70 is being explored for the diagnosis of early-stage breast cancer. In a multicenter prospective trial, researchers are exploring proteomic profiling of exosomes for the prediction of leptomeningeal metastasis (NCT03974204). It should be noted that there are a great number of clinical trials exploring the value of cfDNA as prognostic and predictive factors, and that the majority of cfDNA analysis techniques are assessing all cell free DNA which is generally found in exosomes, thus results from these trials may also be relevant to the field of exosomes.
Conclusions and future perspectives
Studies to understand the role of exosomes in cancer are rapidly expanding. Tumor-derived exosomes transmitted distally contribute to the breast tumor “macroenvironment” by altering tumor-host interactions, while in the breast tumor “microenvironment” BCCs secrete exosomes in a paracrine manner to affect adjacent stromal cells and vice versa. Clinically, exosomes are being explored as nanosized anti-cancer drug carriers to achieve targeted drug delivery, and by manipulating their composition and surface proteins. Furthermore, exosomes have exciting potential as biomarkers with which to screen for early-stage cancer, monitor treatment response and track cancer progression.
Nevertheless, many open questions remain, for example what drives exosome release and exosome contents, whether short-term exposures to exosomes can change disease course or are chronic exposures necessary for evolution/adaptation, and what determines the selectivity of exosomes for specific target cells. It will be important to determine if all exosomes mediate diverse intercellular interactions or if specific subsets exist which are designated to individual ‘tasks’ or specific target cells, and if so, how that is regulated. Currently, the majority of exosome studies are performed using in vitro systems and not in more complex micro- and macro-environments, thereby limiting the understanding of exosome biology. Exosomes are highly heterogenous, with distribution in multiple origins and destinations difficult to be recapitulated in vitro, and hence in vivo studies must be included in future studies. Furthermore, sophisticated technologies and approaches including better exosome markers are required to understand tissue of origin, which will then help to better understand exosome-mediated signaling and to exploit exosomes as biomarkers. Central to the progress this research field is expected to make will be the standardization and validation of isolation methods and nomenclature to allow a more specific understanding of exosomes rather than EVs more broadly. Despite these challenges, we believe that ultimately exosomes may play an important role in the management of breast cancer.
Acknowledgements
This work was in part supported by NIH P30CA047904. The China Scholarship Council and Tsinghua University provided financial support for T.L. This work was supported by the Breast Cancer Research Foundation [A.V.L. and S.O.]; Susan G. Komen Scholar awards [SAC110021 to A.V.L., SAC160073 to S.O.]; S.O. and A.V.L. are Hillman Fellows. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Institutes and Foundations.
Footnotes
Conflicts of interest:
The authors declare no potential conflicts of interest
A Lee and S Oesterreich report grants from The China Scholarship Council and Tsinghua University, Breast Cancer Research Foundation, and Susan G. Komen Scholar awards SAC110021 during the conduct of the study. S. Oesterreich reports grants from AstraZeneca and BCRF, nonfinancial support from Illumina, H3 Biomedicine, Blueprint Medicines, and Zentalis, personal fees from NSABP, and grants from NCI outside the submitted work. No disclosures were reported by the other authors.
References
- 1.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;67:439–48. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 3.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 1983;97:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell 2019;177:428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joyce DP, Kerin MJ, Dwyer RM. Exosome-encapsulated microRNAs as circulating biomarkers for breast cancer. Int J Cancer 2016;139:1443–8. [DOI] [PubMed] [Google Scholar]
- 7.Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res 2016;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW, et al. A New Approach for Loading Anticancer Drugs Into Mesenchymal Stem Cell-Derived Exosome Mimetics for Cancer Therapy. Front Pharmacol 2018;9:1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naseri Z, Oskuee RK, Jaafari MR, Forouzandeh Moghadam M. Exosome-mediated delivery of functionally active miRNA-142–3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int J Nanomedicine 2018;13:7727–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Q, Sun S, Li Z, Yang Q, Li B, Zhu S, et al. Breast cancer-released exosomes trigger cancer-associated cachexia to promote tumor progression. Adipocyte 2019;8:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 2009;458:445–52. [DOI] [PubMed] [Google Scholar]
- 13.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018;19:213–28. [DOI] [PubMed] [Google Scholar]
- 14.Balaj L, Lessard R, Dai L, Cho Y-J, Pomeroy SL, Breakefield XO, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2011;2:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 2014;24:766–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.San Lucas FA, Allenson K, Bernard V, Castillo J, Kim DU, Ellis K, et al. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann Oncol 2016;27:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori MA, Ludwig RG, Garcia-Martin R, Brandão BB, Kahn CR. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab 2019;30:656–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics 2015;13:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles 2016;5:31292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020;182:1044–1061.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tompkins AJ, Chatterjee D, Maddox M, Wang J, Arciero E, Camussi G, et al. The emergence of extracellular vesicles in urology: fertility, cancer, biomarkers and targeted pharmacotherapy. J Extracell Vesicles 2015;4:23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015;161:1046–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006;Chapter 3:Unit 3. 22. [DOI] [PubMed] [Google Scholar]
- 24.Jeppesen DK, Hvam ML, Primdahl-Bengtson B, Boysen AT, Whitehead B, Dyrskjøt L, et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J Extracell Vesicles 2014;3:25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuana Y, Levels J, Grootemaat A, Sturk A, Nieuwland R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J Extracell Vesicles 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol 2015;1295:179–209. [DOI] [PubMed] [Google Scholar]
- 27.Hong C-S, Funk S, Muller L, Boyiadzis M, Whiteside TL. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles 2016;5:29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tatischeff I, Larquet E, Falcón-Pérez JM, Turpin P-Y, Kruglik SG. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J Extracell Vesicles 2012;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardiner C, Ferreira YJ, Dragovic RA, Redman CWG, Sargent IL. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coumans FAW, van der Pol E, Böing AN, Hajji N, Sturk G, van Leeuwen TG, et al. Reproducible extracellular vesicle size and concentration determination with tunable resistive pulse sensing. J Extracell Vesicles 2014;3:25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuana Y, Koning RI, Kuil ME, Rensen PCN, Koster AJ, Bertina RM, et al. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J Extracell Vesicles 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen MH, Beck-Nielsen H, Andersen MN, Handberg A. A flow cytometric method for characterization of circulating cell-derived microparticles in plasma. J Extracell Vesicles 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Zhang H, Jiang X, Qian C, Liu Z, Luo D. Factors involved in cancer metastasis: a better understanding to “seed and soil” hypothesis. Mol Cancer 2017;16:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Zoughbi W, Hoefler G. Tumor macroenvironment: an update. Pathobiology 2020;87:58–60. [DOI] [PubMed] [Google Scholar]
- 36.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018;560:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Li C-W, Chan L-C, Wei Y, Hsu J-M, Xia W, et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res 2018;28:862–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014;25:501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 2014;159:499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K, et al. Mesenchymal Stem Cell-Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer Res 2016;76:5832–44. [DOI] [PubMed] [Google Scholar]
- 41.Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, et al. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol Cancer Res 2017;15:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues G, Hoshino A, Kenific CM, Matei IR, Steiner L, Freitas D, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol 2019;21:1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 2014;14:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018;174:1293–1308.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012;125:5591–6. [DOI] [PubMed] [Google Scholar]
- 46.Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res 2016;18:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol 2018;15:617–38. [DOI] [PubMed] [Google Scholar]
- 48.Wang SE. Extracellular vesicles and metastasis. Cold Spring Harb Perspect Med 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syn N, Wang L, Sethi G, Thiery J-P, Goh B-C. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol Sci 2016;37:606–17. [DOI] [PubMed] [Google Scholar]
- 50.Hannafon BN, Gin AL, Xu Y-F, Bruns M, Calloway CL, Ding W-Q. Metastasis-associated protein 1 (MTA1) is transferred by exosomes and contributes to the regulation of hypoxia and estrogen signaling in breast cancer cells. Cell Commun Signal 2019;17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kia V, Paryan M, Mortazavi Y, Biglari A, Mohammadi-Yeganeh S. Evaluation of exosomal miR-9 and miR-155 targeting PTEN and DUSP14 in highly metastatic breast cancer and their effect on low metastatic cells. J Cell Biochem 2019;120:5666–76. [DOI] [PubMed] [Google Scholar]
- 52.Ding J, Xu Z, Zhang Y, Tan C, Hu W, Wang M, et al. Exosome-mediated miR-222 transferring: An insight into NF-κB-mediated breast cancer metastasis. Exp Cell Res 2018;369:129–38. [DOI] [PubMed] [Google Scholar]
- 53.Fujita Y Interface between normal and transformed epithelial cells: a road to a novel type of cancer prevention and treatment. Cancer Sci 2011;102:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014;26:707–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dutta S, Warshall C, Bandyopadhyay C, Dutta D, Chandran B. Interactions between exosomes from breast cancer cells and primary mammary epithelial cells leads to generation of reactive oxygen species which induce DNA damage response, stabilization of p53 and autophagy in epithelial cells. PLoS One 2014;9:e97580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riches A, Campbell E, Borger E, Powis S. Regulation of exosome release from mammary epithelial and breast cancer cells - a new regulatory pathway. Eur J Cancer 2014;50:1025–34. [DOI] [PubMed] [Google Scholar]
- 57.Takasugi M, Okada R, Takahashi A, Virya Chen D, Watanabe S, Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun 2017;8:15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Gu Y, Cao X. The exosomes in tumor immunity. Oncoimmunology 2015;4:e1027472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ham S, Lima LG, Chai EPZ, Muller A, Lobb RJ, Krumeich S, et al. Breast Cancer-Derived Exosomes Alter Macrophage Polarization via gp130/STAT3 Signaling. Front Immunol 2018;9:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jang J-Y, Lee J-K, Jeon Y-K, Kim C-W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer 2013;13:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang M, Chen J, Su F, Yu B, Su F, Lin L, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer 2011;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker ND, Elias M, Guiro K, Bhatia R, Greco SJ, Bryan M, et al. Exosomes from differentially activated macrophages influence dormancy or resurgence of breast cancer cells within bone marrow stroma. Cell Death Dis 2019;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitai Y, Kawasaki T, Sueyoshi T, Kobiyama K, Ishii KJ, Zou J, et al. DNA-Containing Exosomes Derived from Cancer Cells Treated with Topotecan Activate a STING-Dependent Pathway and Reinforce Antitumor Immunity. J Immunol 2017;198:1649–59. [DOI] [PubMed] [Google Scholar]
- 65.Diamond JM, Vanpouille-Box C, Spada S, Rudqvist N-P, Chapman JR, Ueberheide BM, et al. Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol Res 2018;6:910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ning Y, Shen K, Wu Q, Sun X, Bai Y, Xie Y, et al. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol Lett 2018;199:36–43. [DOI] [PubMed] [Google Scholar]
- 67.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016;16:431–46. [DOI] [PubMed] [Google Scholar]
- 68.Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, et al. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci Rep 2017;7:6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, et al. Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell 2017;170:352–366.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci USA 2017;114:E9066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012;151:1542–56. [DOI] [PubMed] [Google Scholar]
- 72.Baroni S, Romero-Cordoba S, Plantamura I, Dugo M, D’Ippolito E, Cataldo A, et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis 2016;7:e2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol 2015;17:183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalluri R The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016;16:582–98. [DOI] [PubMed] [Google Scholar]
- 75.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011;17:1498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gernapudi R, Yao Y, Zhang Y, Wolfson B, Roy S, Duru N, et al. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res Treat 2015;150:685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Y, Bucan V, Baehre H, von der Ohe J, Otte A, Hass R. Acquisition of new tumor cell properties by MSC-derived exosomes. Int J Oncol 2015;47:244–52. [DOI] [PubMed] [Google Scholar]
- 78.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol 2012;40:130–8. [DOI] [PubMed] [Google Scholar]
- 79.Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr) 2017;40:457–70. [DOI] [PubMed] [Google Scholar]
- 80.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi R, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 2014;7:ra63. [DOI] [PubMed] [Google Scholar]
- 81.Reymond N, d’Água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer 2013;13:858–70. [DOI] [PubMed] [Google Scholar]
- 82.Seton-Rogers S Microenvironment: Endothelial cells create a niche. Nat Rev Cancer 2014;14:298. [DOI] [PubMed] [Google Scholar]
- 83.Di Modica M, Regondi V, Sandri M, Iorio MV, Zanetti A, Tagliabue E, et al. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett 2017;384:94–100. [DOI] [PubMed] [Google Scholar]
- 84.Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding W-Q. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA). Mol Cancer 2015;14:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Che SPY, Park JY, Stokol T. Tissue Factor-Expressing Tumor-Derived Extracellular Vesicles Activate Quiescent Endothelial Cells via Protease-Activated Receptor-1. Front Oncol 2017;7:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weaver O, Leung JWT. Biomarkers and imaging of breast cancer. AJR Am J Roentgenol 2018;210:271–8. [DOI] [PubMed] [Google Scholar]
- 87.Allenson K, Castillo J, San Lucas FA, Scelo G, Kim DU, Bernard V, et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann Oncol 2017;28:741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shimomura A, Shiino S, Kawauchi J, Takizawa S, Sakamoto H, Matsuzaki J, et al. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci 2016;107:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu X, Somlo G, Yu Y, Palomares MR, Li AX, Zhou W, et al. De novo sequencing of circulating miRNAs identifies novel markers predicting clinical outcome of locally advanced breast cancer. J Transl Med 2012;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stevic I, Müller V, Weber K, Fasching PA, Karn T, Marmé F, et al. Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Med 2018;16:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhong Z, Rosenow M, Xiao N, Spetzler D. Profiling plasma extracellular vesicle by pluronic block-copolymer based enrichment method unveils features associated with breast cancer aggression, metastasis and invasion. J Extracell Vesicles 2018;7:1458574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang T, Ning K, Lu T-X, Sun X, Jin L, Qi X, et al. Increasing circulating exosomes-carrying TRPC5 predicts chemoresistance in metastatic breast cancer patients. Cancer Sci 2017;108:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang S-J, Wang D-D, Li J, Xu H-Z, Shen H-Y, Chen X, et al. Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene 2017;623:5–14. [DOI] [PubMed] [Google Scholar]
- 94.Wang X, Zhong W, Bu J, Li Y, Li R, Nie R, et al. Exosomal protein CD82 as a diagnostic biomarker for precision medicine for breast cancer. Mol Carcinog 2019;58:674–85. [DOI] [PubMed] [Google Scholar]
- 95.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013;12:347–57. [DOI] [PubMed] [Google Scholar]
- 96.Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng 2020;4:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yim N, Ryu S-W, Choi K, Lee KR, Lee S, Choi H, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun 2016;7:12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jung KO, Jo H, Yu JH, Gambhir SS, Pratx G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials 2018;177:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohno S-I, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microrna to breast cancer cells. Mol Ther 2013;21:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gomari H, Forouzandeh Moghadam M, Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. Onco Targets Ther 2018;11:5753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kosgodage US, Trindade RP, Thompson PR, Inal JM, Lange S. Chloramidine/Bisindolylmaleimide-I-Mediated Inhibition of Exosome and Microvesicle Release and Enhanced Efficacy of Cancer Chemotherapy. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Monypenny J, Milewicz H, Flores-Borja F, Weitsman G, Cheung A, Chowdhury R, et al. ALIX Regulates Tumor-Mediated Immunosuppression by Controlling EGFR Activity and PD-L1 Presentation. Cell Rep 2018;24:630–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nishida-Aoki N, Tominaga N, Takeshita F, Sonoda H, Yoshioka Y, Ochiya T. Disruption of Circulating Extracellular Vesicles as a Novel Therapeutic Strategy against Cancer Metastasis. Mol Ther 2017;25:181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


