Figure 3: ADK and associated epigenetic mechanisms in pathological conditions.
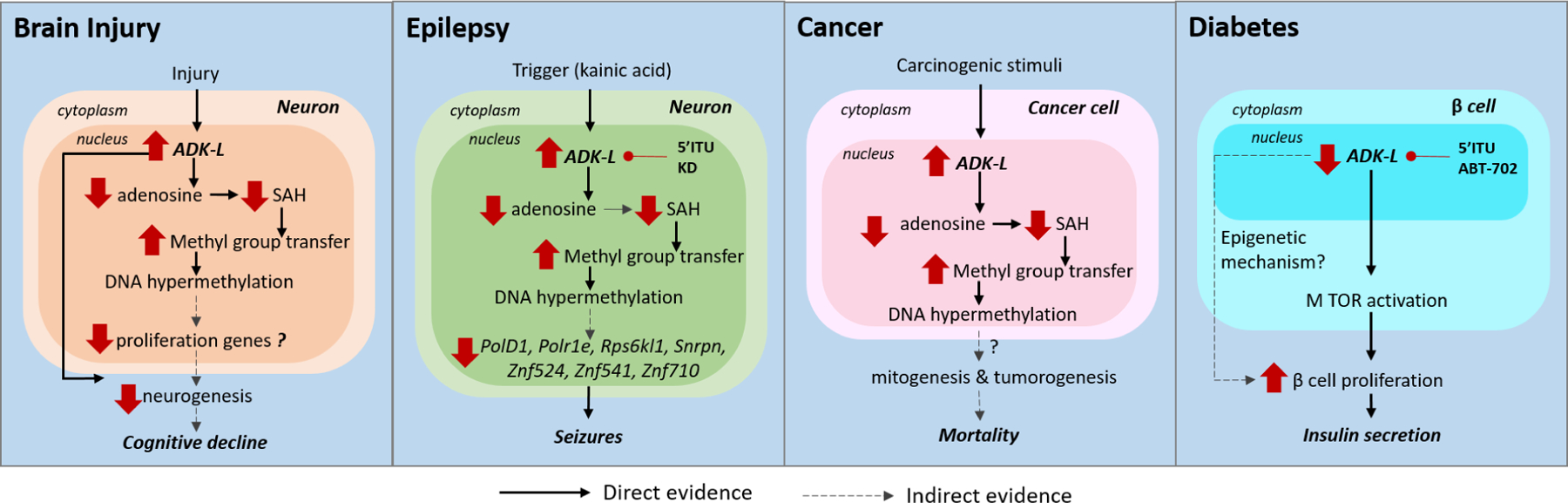
The epigenetic mechanisms mediated by ADK in various pathological conditions are shown. (i) In brain injury, chronic increase in ADK was associated with increased transmethylation leading to DNA hypermethylation and neurogenesis. The links between methylation changes and cognitive decline need to be investigated. (ii) In experimental epilepsy models, a trigger such as kainic acid induces ectopic overexpression of ADK-L and DNA hypermethylation of a network of genes including PolD1, Polr1e, Rps6kl1, Snrpn, Znf524, Znf541, Znf710. ADK inhibition by 5-ITU and ketogenic diet (KD) was able to restore DNA methylation and prevent onset of seizures. (iii) In preclinical cancer models, studies using transgenic mice with ADK deficiency reveals ADK expression changes in tumors may be associated with methylation of mitogenesis and tumorigenesis genes resulting in increased susceptibility to carcinogen responses and associated mortality. (iv) In β-islet cells in the pancreas, ADK inhibition via genetic or pharmacological tools promoted β-cell proliferation and insulin secretion via MTOR activation.
