Abstract
BACKGROUND
Glycemic control is suboptimal in many individuals with type 2 diabetes. Although use of flash continuous glucose monitoring (CGM) has demonstrated A1C reductions in patients with type 2 diabetes treated with a multiple daily injection or insulin pump therapy regimen, the glycemic benefit of this technology in patients with type 2 diabetes using nonintensive treatment regimens has not been well studied.
METHODS
This retrospective, observational study used the IBM Explorys database to assess changes in A1C after flash CGM prescription in a large population with suboptimally controlled type 2 diabetes treated with nonintensive therapy. Inclusion criteria were diagnosis of type 2 diabetes, age <65 years, treatment with basal insulin or noninsulin therapy, naive to any CGM, baseline A1C ≥8%, and a prescription for the FreeStyle Libre flash CGM system during the period between October 2017 and February 2020. Patients served as their own control subject.
RESULTS
A total of 1,034 adults with type 2 diabetes (mean age 51.6 ± 9.2 years, 50.9% male, baseline A1C 10.1 ± 1.7%) were assessed. More patients received noninsulin treatments (n = 728) than basal insulin therapy (n = 306). We observed a significant reduction in A1C within the full cohort: from 10.1 ± 1.7 to 8.6 ± 1.8%; Δ −1.5 ± 2.2% (P <0.001). The largest reductions were seen in patients with a baseline A1C ≥12.0% (n = 181, A1C reduction −3.7%, P <0.001). Significant reductions were seen in both treatment groups (basal insulin −1.1%, noninsulin −1.6%, both P <0.001).
CONCLUSION
Prescription of the flash CGM system was associated with significant reductions in A1C in patients with type 2 diabetes treated with basal insulin or noninsulin therapy. These findings provide evidence for expanding access to flash CGM within the broader population of people with type 2 diabetes.
Large clinical trials have consistently demonstrated that maintaining near-normal glycemia can prevent or delay diabetes-related microvascular and macrovascular disease (1–4). However, suboptimal glycemic control using traditional blood glucose monitoring persists among a substantial number of patients with type 2 diabetes (5,6). As reported by Carls et al. (5), achievement of individualized targets declined from 69.8% in 2010 to 63.8% in 2014, and the percentage of individuals with an A1C >9.0% increased from 12.6 to 15.5% during the same time period. More recently, investigators estimated that more than half (51.5%) of adults with insulin-treated type 2 diabetes have an A1C level >8.0% (7).
Randomized controlled trials have demonstrated that use of flash continuous glucose monitoring (CGM) significantly lowers A1C (8), with reductions in hypoglycemia (9,10) and improved treatment satisfaction (8,9) in people with type 2 diabetes treated with a multiple daily injection (MDI) insulin regimen. However, use of flash CGM has not been well studied in individuals with type 2 diabetes who are treated with less intensive therapy.
The FreeStyle Libre 14-day flash CGM system and the recently cleared (cleared by the U.S. Food and Drug Administration on 15 June 2020) FreeStyle Libre 2 flash CGM system (both Abbott Diabetes Care, Alameda, CA) are the only flash CGM systems available in the United States. An earlier version (a 10-day system) was available from 2017 to 2019. These systems use a single-use, factory-calibrated sensor that continuously measures interstitial glucose levels. By scanning the sensor with a compatible reader or smartphone, users can view their current glucose value, as well as their glucose pattern during the past 8 hours, along with trend arrows indicating the direction and velocity of changing glucose levels. The FreeStyle Libre 2 system functions similarly to the earlier FreeStyle Libre versions and has real-time optional alarms for low and high glucose levels but was not available during the study’s observation period.
We evaluated changes in A1C levels after patients received a prescription for a flash CGM system in a large population of patients with suboptimally controlled type 2 diabetes who were treated with basal insulin or noninsulin therapy.
Research Design and Methods
Design
This retrospective, observational database analysis used a prespecified analysis scheme to assess the impact of a flash CGM system prescription in a large cohort of patients with type 2 diabetes and suboptimal glycemic control who were treated with basal insulin (defined in this study as long-acting, NPH, or a premixed insulin formulation) or noninsulin therapy. The primary outcome measure was change from baseline A1C levels after prescription of the flash CGM system. Secondary outcomes included A1C changes stratified by diabetes therapy and baseline A1C. Inclusion criteria were a diagnosis of type 2 diabetes, age <65 years, naive to any CGM, a baseline A1C ≥8.0%, prescription of a flash CGM system (10- or 14-day) between October 2017 and February 2020, no record of short- or rapid-acting insulin use, presence of baseline A1C test within the 180 days before or including the flash CGM prescription date, and presence of a post-observation A1C value between 60 and 300 days after the CGM prescription date.
Eligible patients were identified using data obtained from the IBM Explorys database, which includes de-identified electronic health record data that reside in a highly secure, Cloud-based, Health Insurance Portability and Accountability Act–enabled platform. The database contains underlying patient-level data for a defined population based on a set of selection criteria. Available data included demographic information, medical records, laboratory data, and pharmacy prescriptions. Available data were extracted for study on 29 April 2020.
International Classification of Diseases, 9th and 10th revisions (ICD-9 and ICD-10), billing codes and Systematized Nomenclature of Medicine–Clinical Terms (SNOMED CT) codes were used to identify patients with diagnosed type 2 diabetes. Diabetes type was determined from the closest relevant diagnosis before the flash CGM prescription. In the rare case that the closest encounter had codes related to both type 1 and type 2 diabetes, the patient was excluded. Patients with a gestational diabetes diagnosis in the 6 months before their flash CGM prescription were also excluded.
ICD-9, ICD-10, and SNOMED CT codes were also used to identify the prevalence of comorbidities within the study cohort. Comorbidities were identified by the presence of a related code in the medical encounters at any time from the beginning of each patient’s data availability through the day of acquiring the prescription for flash CGM.
National Drug Code (NDC) data were used to identify patients who were treated with basal insulin or noninsulin therapy and who had a record of a flash CGM prescription in the same time period. Code sets were determined by medical expert review.
To ensure that patients were naive to CGM, we excluded those with evidence of prior CGM prescription, including sensor, transmitter, or receiver, identified via either NDC codes or Healthcare Common Procedure Coding System codes. Prescriptions for the flash CGM system were identified through either the presence of associated NDC codes or the appearance of the system name in the prescription description field.
Outcomes
The primary outcome of the study was difference in A1C after acquisition of a prescription for a flash CGM compared with before receiving the prescription. Baseline A1C was defined as the value within 180 days pre-index closest to the flash CGM prescription date, including the CGM prescription date, and post-flash CGM A1C was defined as the value closest to 180 days post-prescription and within 60–300 days after the flash CGM prescription.
Secondary analyses included changes in A1C by treatment (insulin vs. noninsulin) and baseline A1C stratified into three subgroups: ≥8.0 to <10.0, ≥10.0 to <12.0, and ≥12.0%. An exploratory analysis was performed on the full spectrum of A1C baseline values, including people with A1C <8% who were excluded from the primary cohort analysis.
Statistical Analysis
The analysis was structured as patient-as-own-control. All changes in A1C values were evaluated with paired t tests, and changes in A1C categories were evaluated with χ2 tests. Subgroup analyses are presented uncorrected for multiple comparisons. RStudio, v. 1.0.153 (Boston, MA), with R, v. 3.4.0, software was used for statistical analysis.
Results
Patient Characteristics
From the IBM Explorys database, we identified a cohort of 1,034 patients with type 2 diabetes for assessment. Most patients were >50 years of age, had a baseline A1C of ≥8.0 to <10.0%, and were treated with noninsulin therapy. The majority of patients had hypertension and dyslipidemia, and more than half had a BMI >30 kg/m2. Premixed insulin formulations were rare, with only 2.1% of patients using a premixed insulin as their only insulin therapy. Patient characteristics and diabetes medications are reported in Table 1.
TABLE 1.
Patient Characteristics (N = 1,034)
| Age, years | 51.6 ± 9.2 |
| Male sex | 50.9 |
| Baseline A1C | |
| ≥8.0 to <10.0% (n = 576, mean 8.8 ± 0.6%) | 55.7 |
| ≥10.0 to <12.0% (n = 277, mean 10.9 ± 0.6%) | 26.8 |
| ≥12.0% (n = 181, mean 13.1 ± 0.9%) | 17.5 |
| Baseline treatment type | |
| Basal insulin (long-acting, NPH, or premixed) | 29.6 |
| Noninsulin | 70.4 |
| Treatment regimens for noninsulin users | |
| No diabetes medications | 22.4 |
| 1 diabetes medication | 22.4 |
| 2 diabetes medications | 27.0 |
| ≥3 diabetes medications | 28.2 |
| Comorbidities | |
| Lipid disorder | 82.2 |
| Hypertension | 77.3 |
| Obesity | 62.1 |
| Liver disease | 16.3 |
| Heart failure | 7.7 |
| Ethnicity* | |
| Hispanic | 2.9 |
| Non-Hispanic | 68.4 |
| Other | 6.4 |
| Unavailable or declined to answer | 22.3 |
| Race* | |
| African American | 22.1 |
| Asian | 1.0 |
| Caucasian | 59.0 |
| Hispanic/Latino | 0.8 |
| Multiracial | 0.8 |
| Other | 1.8 |
| Unavailable or declined to answer | 14.5 |
Data are percentages, except age, which is mean ± SD.
*Ethnicity and race were self-reported by patients.
Outcomes
At study end point (mean follow-up 159 days), we observed a significant A1C reduction of 1.5 ± 2.2 percentage points within the full cohort (Figure 1).
FIGURE 1.
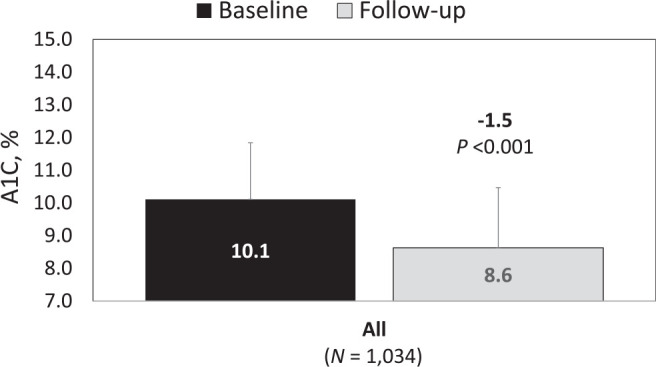
Change in A1C after flash CGM prescription.
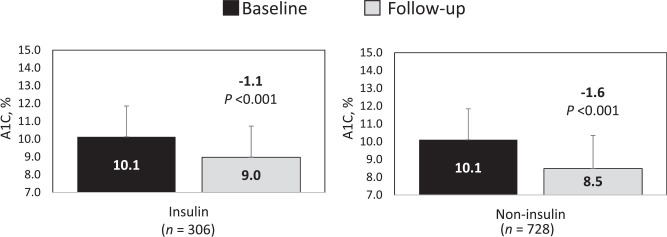
In a subgroup analysis by insulin versus noninsulin therapy, patients treated with noninsulin therapy showed a notably greater A1C reduction (1.6 ± 2.3 percentage points) compared with those treated with basal insulin (1.1 ± 1.9 percentage points) despite starting at similar A1C levels before the flash CGM prescription (Figure 2).
FIGURE 2.
A1C changes by baseline treatment.
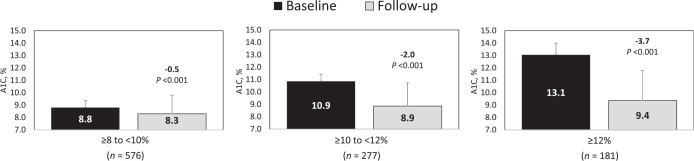
The largest A1C reductions were observed in patients with a baseline A1C ≥12%, followed by those with a baseline A1C ≥10 to <12% (Figure 3).
FIGURE 3.
A1C changes by baseline A1C value.
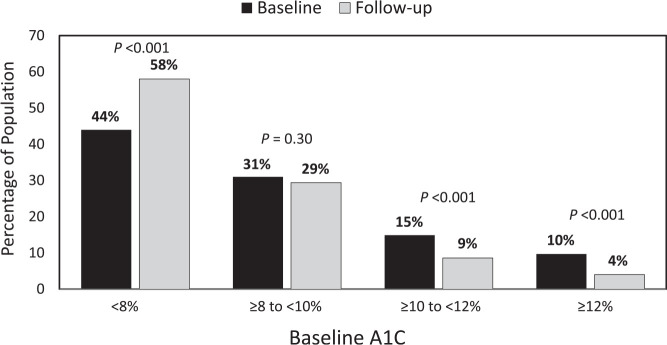
An exploratory analysis was performed for the full spectrum of A1C baseline values, including people whose well-controlled A1C (<8%) excluded them from the primary cohort (n = 1,859) (Figure 4). We observed a shift in the distribution of A1C values resulting in an increase in the percentage of patients who achieved A1C values <8.0% at end point (P <0.001). Reductions in the percentage of patients with an A1C ≥10.0 to <12.0 and ≥12.0% were also observed (both P <0.001). A narrower targeted comparison showed an increase in patients with an A1C <7%, from 21.7 to 32.2% (P <0.001).
FIGURE 4.
Change in A1C distribution after prescription of flash CGM.
Discussion
The use of flash CGM was recently shown to lower A1C in adults with type 2 diabetes treated with an MDI insulin regimen (8). To our knowledge, this is the first study to demonstrate the glycemic benefits of flash CGM use in a large population of people with type 2 diabetes treated with basal insulin or noninsulin therapies. Our findings showed a significant association between prescription of flash CGM and reductions in A1C. It was particularly interesting that patients treated with noninsulin therapies achieved notably greater A1C reductions compared with those on basal insulin therapy despite having similar baseline A1C levels.
As expected, patients with the highest A1C at baseline (≥12.0%) experienced the largest reduction in A1C; the percentage of patients with an A1C ≥12.0% at baseline decreased by more than half after flash CGM prescription. We also saw a significant increase in the percentage of patients who achieved A1C levels <8 or <7%.
An important strength of our study was its use of the IBM Explorys database, which allowed us to track our cohort over time to detect changes in A1C levels before and after prescription of the flash CGM device without reliance on self-reported data. Moreover, our findings are potentially generalizable to the vast majority of individuals with type 2 diabetes who are treated with nonintensive therapy and have poorly controlled A1C. Recent data show that 14.1% of individuals with diabetes are treated with basal-only insulin, and more than half (51.7%) take oral or noninsulin injectable medications only (11).
A notable limitation is that the observational study design did not allow us to evaluate the degree or significance of A1C change compared with no CGM system prescription. Nor were we able to confirm acquisition of the system; the dataset only provided information on when patients received their prescriptions. Patients’ A1C history outside our study window also was not captured. Additionally, we were not able to determine patients’ persistence in monitoring or use of their glucose data. Moreover, our findings cannot be generalized to elderly patients, a population that is at higher risk for severe hypoglycemia (12–14) and is less likely to use technology than younger patients with diabetes (15).
Nevertheless, our findings suggest that expanding insurance coverage of flash CGM to include patients with type 2 diabetes who are generally not considered to be eligible may help improve glycemic control within the larger type 2 diabetes population. However, changes in current eligibility criteria may occur sooner than expected. As a consequence of the coronavirus disease 2019 pandemic, an increasing number of clinicians are using telemedicine and digital diabetes technologies to provide essential care to minimize face-to-face clinic visits.
Flash CGM systems give patients the ability to automatically transfer glucose data to clinicians, who then use the data to provide guidance and therapy recommendations via remote clinical consults (16). Our findings of improved glycemic control and recent evidence demonstrating reductions in diabetes-related hospitalizations and health care resource utilization associated with flash CGM use (17) suggest that expanding CGM insurance coverage to type 2 diabetes patients treated with less intensive therapy would improve clinical outcomes.
Conclusion
In this real-world, retrospective, observational study, we observed significant A1C reductions within a large cohort of adult patients with type 2 diabetes who were treated with basal insulin or noninsulin therapy and had poorly controlled A1C at baseline after they received a prescription for a flash CGM system. Additional studies are needed to further elucidate patient behaviors relevant to monitoring persistence and use of data, as well as the impact of flash CGM on clinical outcomes and health care resource utilization within this population.
Article Information
Acknowledgments
The authors thank Chris Parkin of CGParkin Communications for providing medical writing support and Dr. Naunihal Virdi and Laura Brandner, both of Abbott Diabetes Care, for input and review of the manuscript.
Funding
This research was funded by Abbott Diabetes Care.
Duality of Interest
E.E.W. has received consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Mannkind, Merck, Sanofi US, and Voluntis and has been a speaker for Abbott, Bayer, Boehringer Ingelheim, and Eli Lilly. M.S.D.K., I.J.R., and Y.N. are employed by Abbott. E.M. has received consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, and Sanofi US and has been a speaker for Abbott, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
Author Contributions
All authors were responsible for designing the study. E.E.W. and E.M. wrote the manuscript. M.S.D.K., I.J.R., and Y.N. performed the data analysis. All authors reviewed/edited the manuscript. E.E.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
Portions of these data were presented as an abstract at the American Diabetes Association’s virtual 80th Scientific Sessions, 12–16 June 2020.
References
- 1. UK Prospective Diabetes Study Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 3. Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 4. Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carls G, Huynh J, Tuttle E, Yee J, Edelman SV. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther 2017;8:863–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stone MA, Charpentier G, Doggen K, et al.; GUIDANCE Study Group . Quality of care of people with type 2 diabetes in eight European countries: findings from the Guideline Adherence to Enhance Care (GUIDANCE) study. Diabetes Care 2013;36:2628–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lauffenburger JC, Lewey J, Jan S, Lee J, Ghazinouri R, Choudhry NK. Association of potentially modifiable diabetes care factors with glycemic control in patients with insulin-treated type 2 diabetes. JAMA Netw Open 2020;3:e1919645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care 2019;42:1178–1184 [DOI] [PubMed] [Google Scholar]
- 9. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of flash glucose-sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther 2017;8:573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017;8:55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Diabetes Association . Fast facts: data and statistics about diabetes. Available from https://professional.diabetes.org/sites/professional.diabetes.org/files/media/sci_2020_diabetes_fast_facts_sheet_final.pdf. Accessed 20 May 2020
- 12. Meneilly GS, Cheung E, Tuokko H. Counterregulatory hormone responses to hypoglycemia in the elderly patient with diabetes. Diabetes 1994;43:403–410 [DOI] [PubMed] [Google Scholar]
- 13. Meneilly GS, Tessier D. Diabetes in the elderly. Diabet Med 1995;12:949–960 [DOI] [PubMed] [Google Scholar]
- 14. Schütt M, Fach EM, Seufert J, et al.; DPV Initiative and the German BMBF Competence Network Diabetes Mellitus . Multiple complications and frequent severe hypoglycaemia in ‘elderly’ and ‘old’ patients with type 1 diabetes. Diabet Med 2012;29:e176–e179 [DOI] [PubMed] [Google Scholar]
- 15. Czaja SJ, Charness N, Fisk AD, et al. Factors predicting the use of technology: findings from the Center for Research and Education on Aging and Technology Enhancement (CREATE). Psychol Aging 2006;21:333–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levine BJ, Close KL, Gabbay RA. Reviewing U.S. connected diabetes care: the newest member of the team. Diabetes Technol Ther 2020;22:1–9 [DOI] [PubMed] [Google Scholar]
- 17. Miller E, Kerr MSD, Roberts GJ, Souto D, Nabutovsky Y, Wright E Jr. FreeStyle Libre system use associated with reduction in acute diabetes events and all-cause hospitalizations in patients with type 2 diabetes without bolus insulin [Abstract]. Diabetes 2020;69(Suppl. 1):85-LB [Google Scholar]