Microvascular dysfunction has been implicated in many diseases such as stroke and diabetes. In addition to the microvascular endothelial cell (EC), the pericyte, a perivascular cell that adheres to the abluminal side of the EC may also be important to ensure proper microvascular function. As a prominent perivascular cell, the pericyte has garnered increasing attention for its multiple functional aspects, especially the pericyte of central nervous system (Yemisci et al., 2009; Armulik et al., 2010; Gaceb et al., 2018). The integrity of the blood-brain barrier (BBB), regulation of cerebral blood flow, and proper functioning of the glymphatic and immune defense systems may all be dependent on the coordination between brain pericytes and their neighboring cells (Yemisci et al., 2009; Armulik et al., 2010; Gaceb et al., 2018). Since the brain communicates with all peripheral organs, pericyte dysfunction in brain and in peripheral organs may, respectively, adversely impact peripheral organs and brain. Thus, pericyte behavior may provide potential links in the communication axis of brain and peripheral organs. In this perspective, we discuss associations of pericyte functions and pathologies between brain and selective peripheral organs, and elucidation of which may lead to novel therapeutic applications.
Pericyte functions and pathologies in brain and peripheral organs: In brain where pericytes are sandwiched between ECs and astrocytes, a crucial function of pericytes is to maintain the integrity of BBB (Armulik et al., 2010). Pericytes not only preserve the expression of tight junctions between ECs, but also maintain low vesicular trafficking (also known as transcytosis) within the ECs, both of which are essential to ensure an intact functional BBB (Armulik et al., 2010). Pericytes may also regulate the cerebral blood flow at the level of capillaries, though this function is debated (Yemisci et al., 2009). The “no-flow” phenomenon present in the setting of ischemic stroke that occurs after reperfusion of the occluded artery, the downstream capillaries remain “closed” with no blood flow passing through (Yemisci et al., 2009). One possible contribution to no-flow is that the pericytes die in rigor and thereby permanently constrict the underlying capillaries (Yemisci et al., 2009). In heart, a similar finding is seen in myocardial infarction where pericytes constrict the cardiac capillaries despite the reperfusion of the upstream coronary arteries. A recent study showed that brain stroke may lead to cardiac deficit without previous cardiac dysfunction (Chen et al., 2017). Pericyte dysfunction may participate in both primary brain ischemia and the ensuing cardiac deficit secondary to stroke. Therefore, therapies that systemically relax the pericytes may not only attenuate the “no flow” damage of the brain but also attenuate the potential injury to the heart and other organs. Another example is type 2 diabetes mellitus (T2DM), a disease that targets microvessels in various tissue. Pericytes regulate blood flow in the pancreatic islet (Almaça et al., 2018). As T2DM progresses, the density of pericytes in capillaries decrease and lose their control of islet blood flow, resulting in reduced insulin release to metabolize glucose. Hyperglycemia which causes pericyte loss in the pancreas may also weaken the pericytes of the brain, and subsequently compromise their functions in regulating BBB, neurovascular coupling, and waste clearance. The pericytes in certain high-pressure areas of brain may more likely be damaged and increase the risk of hemorrhagic stroke from brain vessel rupture. In this regard, pericyte loss in pancreatic islet and in the central nervous system contributes to the vulnerability of brain to future insult, which may partially explain why T2DM patients are more susceptible to brain diseases such as stroke, dementia and Alzheimer’s disease. Additionally, amylin, an amyloid derived from pancreatic β-cells, accumulates in the pancreas and brain hippocampus in patients with T2DM. Amylin forms inclusion bodies in brain pericytes and induces cell dysfunction that is related to the progression of Alzheimer’s disease (Schultz et al., 2017). Thus, pericytes maybe an amylin target and thereby cause cognitive decline in the setting of T2DM. Measurement of the amylin deposition in pancreatic pericytes may be helpful to investigate the association of these two diseases. Likewise, if amylin deposition in pericytes leads to pericyte dysfunction, it may also be related to stroke onset in T2DM. Therefore, the level of amylin in brain and pancreatic pericytes should also be evaluated in stroke patients with T2DM. All these findings suggest that pericytes from different tissues interact and may underlie the same pathology. Extended evaluation of pericyte levels and function of various tissues may be conducive to guide the therapies to benefit both primary and secondary diseases.
Stem cell properties of pericytes: A potential way for pericytes from different tissues to communicate relates to their proposed stem cell-like properties. Crisan et al. (2008) showed that the perivascular cells, especially pericytes with CD146, NG2 and PDGFR-beta labeling express mesenchymal stem cells markers both in vitro and in vivo. After their injection into the injured muscle of immunosuppressed mice, cultured pericytes isolated from various tissues can all give rise to myofibers (Crisan et al., 2008). This suggests that pericytes at one site might be able to participate in tissue regeneration at the other (Crisan et al., 2008). However, there are some concerns with this conclusion. First, the cultured pericytes once out of their endogenous niche may not fully represent the resident pericytes. Therefore, the fate of the resident pericytes in the area of the injured muscle should also be traced. Second, the pericyte markers used in the study can also label vascular smooth muscle cells, and thus the origin of the regenerated myofiber may be the cultured vascular smooth muscle cells. Third, the mouse line used in the study is an immunodeficient mouse which may allow the transplanted cells to grow independently of their stem cell potential due to the low immune environment. Therefore, investigation of the cultured pericytes in immunocompetent mice is warranted. In contrast, Guimaraes-Camboa et al. (2017) showed that pericytes, identified by Tbx-18 staining, do not undergo obvious differentiation in vivo during aging and pathological conditions. The Tbx-18 positive cells can differentiate into adipocytes, osteocytes and chondrocytes in vitro, which is consistent with the results of Crisan et al. (2008) study. However, the Guimaraes-Camboa study did not observe further differentiation of pericytes in vivo. The possible reasons for the discrepancies maybe the respective usage of different pericyte markers in the two studies. As pericytes are very heterogeneous, the difference in markers may identify a different pericyte subtype, and therefore, have distinctive properties. In addition, Crisan et al. (2008) observed the growth of the transplanted pericytes, whereas Guimarães-Camboa et al. (2017) studied the resident pericytes. Thus, it is likely that the two studies focused on the different pericytes subtypes, and thereby yield different results. From another perspective, however, these two studies may also complement each other. It is possible that the interactions between the exogenous pericytes and resident pericytes are more important and have synergistic effects for tissue regeneration. Therefore, the studies regarding the stem cell potential of pericytes should be more specific to certain pericyte subtypes, but at the same time, the communications among different subtypes should also be investigated.
Pericytes secrete microvesicles (MVs): Pericytes can secrete MVs whose content depends on conditions and types of stimulation. For example, platelet derived growth factor stimulate pericytes to secrete abundant growth factors and angiogenic factors encapsulated in MVs by which pericytes support normal neuronal activity (Gaceb et al., 2018). Pericytes may also secrete MVs with anti-inflammatory factors in the setting of an overreactive immune response. In contrast, pericytes secrete MVs containing elevated amounts of mainly inflammatory cytokines, which regulate neuroinflammation in response to lipopolysaccharide stimulation (Gaceb et al., 2018). In central nervous system disease, pericytes may use MVs to participate in tissue remodeling by acting as immune cells to promote inflammation during the early phase of the disease and limit inflammation in the recovery phase, during which pericytes may generate MVs containing growth factors to promote angiogenesis and neurogenesis. In this regard, MVs derived from pericytes are efficient tools for pericytes to exert a multiplicity of specific functions in physiology and pathology.
Brain injury can elicit specific MVs generated by astrocytes and neurons (Zhou et al., 2018). These MVs may be immune stimulatory for peripheral tissues and amplifythe immune response to cause damage. Moreover, brain-derived MVs (BDMVs) enriched with phospholipid can trigger systemic coagulopathy once released in peripheral circulation (Zhou et al., 2018). Lactadherin (also known as milk fat globule-epidermal growth factor 8), is a powerful scavenger for MVs mainly produced by monocytes (Zhou et al., 2018). Lactadherin is not only able to attenuate coagulopathy and suppress inflammation after brain injury, but also preserves the BBB (Zhou et al., 2018). In response to brain injury, pericytes may be a source of BDMVs, as noted above. But more importantly, pericytes are also a source of lactadherin (Vanlandewijck et al., 2018). As pericytes are dying or dysfunctional in disease, their ability to secrete lactadherin may be compromised. This may be pronounced in lung, as lactadherin is predominantly secreted from pericytes in the pulmonary microvascular bed (Vanlandewijck et al., 2018). Severe pericyte loss in brain injury will first increase the leakage of BDMVs via the compromised BBB to induce systemic inflammation and coagulopathy. Deficiency of lung pericytes may further accelerates the progression of pulmonary embolism. Therefore, individuals with genetic lactadherin deficiency or defects in its parent cells such as pericytes may be more prone to develop BDMVs-induced complications. Prophylactically administering lactadherin or boosting its endogenous production to people in high thrombotic risk may be a therapeutic consideration.
Pericyte-related microRNAs (miRs): A plethora of miRs are expressed in pericytes and their levels are altered in pathologies (Larsson et al., 2009). Yet, their functions in pericytes are largely unknown. The role of pericyte generated miRs are likely versatile, as pericytes may provide a compensatory therapeutic response to insult and disease, or detrimental factors, respectively. Pericyte miRs released to the extracellular space and pericyte miRs encapsulated within MVs may function differently, and their respective levels in the circulation may differ in health and disease. Alterations of the miRs within pericytes may be determined by the specific response of pericytes to progression of disease and injury. Thus, miRs may provide biomarkers reflecting the progression of disease.
As specified above, pericyte behavior may provide potential links in the communication axis of brain and peripheral organs, and miRs may serve as important mediators of this communication process. For example, miR-145 is selectively expressed by pericytes in the microvessels of brain and kidney (Larsson et al., 2009). In response to hypoxia, pericytes significantly increase miR-145 expression. However, reducing miR-145 rather than increasing it may promote neurorestoration in stroke animal model (Cui et al., 2016). In this regard, the pericyte-derived miR-145 may exert unfavorable effects in stroke. If the miR-145 in kidney pericytes act in this adverse way, it could possibly induce kidney deficits which is a common complication after stroke. Thus, therapies that target pericytes in both brain and kidney may be beneficial. Alternatively, it is also possible that the alteration of pericyte generated miRs are organ-dependent, and the miRs that are beneficial to one organ may possibly be ineffective or even harmful for others. Therefore, broad investigations of pericyte generated miRs in different organs of various diseases are needed and may be helpful to design therapeutic strategies.
In summary, pericytes are ubiquitous and heterogenous. The functions of the various subtypes of pericytes may not be completely isolated but complement and amplify each other. Pericytes of brain and peripheral organs could be the common target for certain pathologies. Pericytes as well as pericyte-derived MVs and miRs may all participate in the communication axis between brain and peripheral organs (Figure 1). Therefore, future studies of the pericyte should not be confined to one site but be extended to the entire body as a whole, which, hopefully, may provide a more complete view for its potential therapeutic translation.
Figure 1.
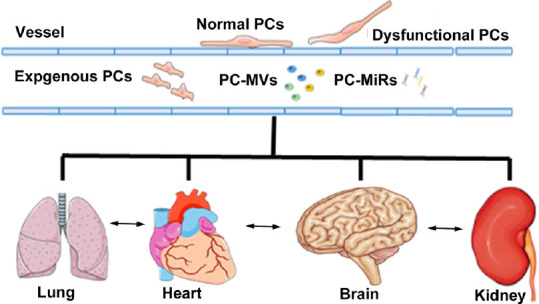
PCs participate in the communications among organs.
PCs reside in the microvascular beds of multiple organs. Dysfunctional PCs in one organ may render the PCs of other organs more susceptible to pathological conditions. PCs secrete microvesicles and microRNAs which mediate interorgan interactions in both health and disease. Therefore, exogenous administration of PCs or PC derivatives such as microvesicles and microRNAs may provide therapeutic benefit not only for the primary injury but also for the secondary complications as well as promote a restorative environment which facilitates systemic recovery. PC: Pericytes; PC-miRs: pericyte-derived microRNAs; PC-MV: pericyte-derived microvesicles.
This work was supported by the National Heart, Lung, and Blood Institute R01HL143432 (to JC).
Footnotes
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Almaça J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab. 2018;27:630–644.e634. doi: 10.1016/j.cmet.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, Yu P, Chopp M. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res. 2017;8:374–385. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301, 313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Cui C, Ye X, Chopp M, Venkat P, Zacharek A, Yan T, Ning R, Yu P, Cui G, Chen J. miR-145 regulates diabetes-bone marrow stromal cell-induced neurorestorative effects in diabetes stroke rats. Stem Cells Transl Med. 2016;5:1656–1667. doi: 10.5966/sctm.2015-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaceb A, Özen I, Padel T, Barbariga M, Paul G. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J Cereb Blood Flow Metab. 2018;38:45–57. doi: 10.1177/0271678X17719645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guimarães-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20:345–359e5. doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson E, Fredlund Fuchs P, Heldin J, Barkefors I, Bondjers C, Genové G, Arrondel C, Gerwins P, Kurschat C, Schermer B, Benzing T, Harvey SJ, Kreuger J, Lindahl P. Discovery of microvascular miRNAs using public gene expression data: miR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med. 2009;1:108. doi: 10.1186/gm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz N, Byman E, Fex M, Wennström M. Amylin alters human brain pericyte viability and NG2 expression. J Cereb Blood Flow Metab. 2017;37:1470–1482. doi: 10.1177/0271678X16657093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 11.Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Cai W, Zhao Z, Hilton T, Wang M, Yeon J, Liu W, Zhang F, Shi FD, Wu X, Thiagarajan P, Li M, Zhang J, Dong JF. Lactadherin promotes microvesicle clearance to prevent coagulopathy and improves survival of severe TBI mice. Blood. 2018;131:563–572. doi: 10.1182/blood-2017-08-801738. [DOI] [PMC free article] [PubMed] [Google Scholar]


