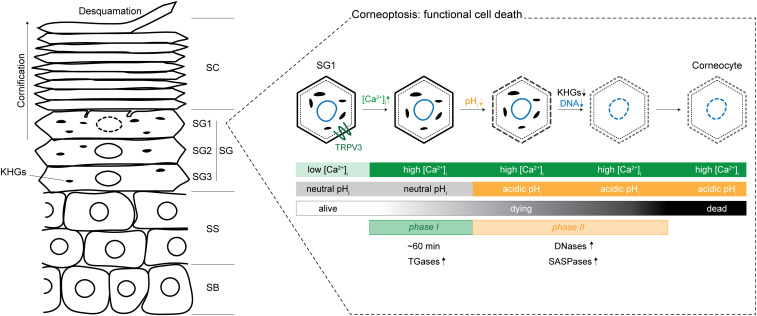
As the mammalian body’s largest organ, the skin is the first defense against a host of environmental insults. Specifically, the outermost layer of the skin, the stratum corneum (SC), provides much of the essential barrier function of the epidermis, protecting the body’s surface from mechanical force, water loss, pathogens, toxins, and allergens (1). Epithelial cells of the skin, called keratinocytes, undergo various sequential stages of differentiation on their way to this outermost layer of the stratified epithelium. Proliferative keratinocytes originate in the stratum basale (SB) and first differentiate into the stratum spinosum (SS) layers (2, 3). They continue differentiating, forming the three layers of the stratum granulosum (SG3, SG2, and SG1), with a tight junction-mediated barrier forming in SG2. Finally, SG1 cells undergo a very specific kind of cell death, beginning the process of cornification and leading to the formation of the SC, layers of dead keratinocytes and the functional barrier of the skin (4, 5). This differentiation step is perhaps one of the most interesting, as the remnants of this mode of cell death (corneocytes) remain functional and essential to the skin barrier. This complex form of keratinocyte death involves degradation of membraneless organelles called keratohyalin granules (KHGs), formation of the cornified envelope which replaces the plasma membrane, and degradation of the nucleus (Fig. 1) (1, 4). However, the exact cellular and molecular mechanisms underpinning this unique process of cell death are still not fully understood. Matsui et al. (6) combine elegant in vivo and in vitro experiments to dissect the process of SG1 cell death they coin “corneoptosis,” the initial step in cornification.
Fig. 1.
Schematic of the layers of the epidermis and phases of functional cell death, corneoptosis. Epidermal cells differentiate vertically through the stratified layers of the epidermis, the SB, SS, SG, and SC. The SG is made up of three layers, SG3, SG2, and SG1, with SG1 cells undergoing functional cell death, corneoptosis. In phase I of this process, there is sustained high [Ca2+]i at neutral pH for about 60 min, which may activate transglutaminases (TGases). In phase II, the high [Ca2+]i is sustained, and pH becomes acidic, which may activate DNases (DNase1L1 and DNase2) and SASPase. KHGs are eliminated, and nuclear DNA is completely degraded, completing the cell death process leading to a functional corneocyte. Corneocytes mature within the SC and are finally shed off in a process called desquamation. Adapted with permission from ref. 6.
First, the authors (6) deeply characterize the temporal framework of SG1 cell death, defined by changes in intracellular calcium concentration ([Ca2+]) and pH. To do this, they combined multiple fluorescent reporters in vivo, including pH-sensitive and -insensitive [Ca2+] indicators and a newly developed ratiometric pH probe. Using these fluorescent reporters in the SG1 cells of live mice, they find that corneoptosis consists of two stereotyped and temporally distinct phases. Building off recent work (7), the authors show that SG1 cells in phase I show high [Ca2+] with a neutral pH for about 60 min, followed closely by cells in phase II sustaining high [Ca2+] with pH dropping rapidly, leading to an acidic pH (6).
Next, the authors (6) develop a way to specifically isolate and culture SG1 cells in vitro, allowing them to subject these cells to different calcium and pH conditions to interrogate the mechanisms coordinating the cellular changes involved in SG1 cell death. Through these experiments, they demonstrate that exposure to high extracellular calcium in culture media (analogous to phase I) leads to an intracellular calcium spike and cell membrane degradation. Simulating phase II, exposure to both high extracellular calcium and low pH caused and was necessary for two additional changes in this unique cell death process, the degradation of KHGs and degradation of nuclear DNA.
From these data, the authors (6) suggest specific biological roles for phase I and phase II of SG1 cell death, clarifying the sequential steps of corneoptosis. First, what is the role of sustained high [Ca2+] with neutral pH in phase I of SG1 cell death? Transglutaminases are calcium-dependent enzymes involved in the formation of the cornified envelope, an insoluble mix of cross-linked proteins and lipids that replaces the plasma membrane in corneocytes (1). Matsui et al. (6) suggest that the sustained elevation in [Ca2+] in phase I could be activating transglutaminases 3 and 5, leading to formation of the cornified envelope. In the future, it will be important to further understand exactly when and how phase I is triggered. It is unclear whether demand from delaminating SC cells, a signal from the lower layers, neighbor−neighbor interactions within SG1, or a temporal maturation of these cells could be implicated in triggering phase I.
Second, why is there a rapid decrease in intracellular pH while high [Ca2+] is maintained in phase II? The in vitro experiments from the authors (6) demonstrating that both acidic pH and high [Ca2+] are necessary for KHG degradation and complete nuclear DNA degradation suggest that phase II temporally regulates these processes. KHGs are liquid-like membraneless organelles composed of protein deposits of keratin 1, keratin 10, and profilaggrin (a precursor of filaggrin) (8). It is thought that various proteases, such as skin aspartic protease (SASPase) and endopeptidase-1 (PEP-1), cleave profilaggrin to filaggrin and allow it to bundle keratin filaments into a scaffold (4). Both keratins and SASPase may be pH sensitive and could explain the authors’ observation that a rapid drop of pH in phase II leads to the degradation of KHGs (9–11). Additionally, Matsui et al. (6) show that complete nuclear degradation, a step that must conclude before cells reach the SC layer, is dependent on the rapid drop in pH observed during phase II. The authors suggest this could be carried out by DNase1-like 2 (DNase1L2) and DNase2, which are DNases that have been shown to be optimal at acidic pH and active in SG1 cells (12–14).
Finally, the authors (6) ask how [Ca2+] and pH are molecularly regulated. Using a newly developed method for a whole-cell patch-clamp assay in SG1 cells and drug treatments targeting highly expressed transient receptor potential (TRP) channels, they show that TRPV3 is functional in SG1 cells. With TRPV3 knockout mice and live imaging, the authors demonstrate that TRPV3 is essential in regulating the timing of phase II onset as well as the spatial and temporal homeostasis of corneoptosis. Interestingly, Matsui et al. show that TRPV3 is temperature sensitive in SG1 cells, opening the question of how TRPV3 is activated during corneoptosis, while also leaving the door open to other molecules playing a role in SG1-specific [Ca2+] elevation.
Taken together, this work defines two phases of SG1 cell death and implicates TRPV3 mechanistically in its temporal regulation. This paper builds nicely off of recent in vivo work implicating changes in calcium and pH in the terminal differentiation process and integrates the field’s existing understanding of this process into a temporal framework (7, 8). Ultimately, these impactful insights, combined with a broadly applicable toolset, will allow the field to further interrogate the molecular mechanisms and spatiotemporal coordination of a unique mode of cell death, corneoptosis.
Footnotes
The authors declare no competing interest.
See companion article, “A unique mode of keratinocyte death requires intracellular acidification,” 10.1073/pnas.2020722118.
References
- 1.Candi E., Schmidt R., Melino G., The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Eckert R. L., Structure, function, and differentiation of the keratinocyte. Physiol. Rev. 69, 1316–1346 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Watt F. M., Terminal differentiation of epidermal keratinocytes. Curr. Opin. Cell Biol. 1, 1107–1115 (1989). [DOI] [PubMed] [Google Scholar]
- 4.Matsui T., Amagai M., Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 27, 269–280 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Eckhart L., Lippens S., Tschachler E., Declercq W., Cell death by cornification. Biochim. Biophys. Acta 1833, 3471–3480 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Matsui T., et al., A unique mode of keratinocyte death requires intracellular acidification. Proc. Natl. Acad. Sci. U.S.A., 10.1073/pnas.2020722118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murata T., et al., Transient elevation of cytoplasmic calcium ion concentration at a single cell level precedes morphological changes of epidermal keratinocytes during cornification. Sci. Rep. 8, 6610 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quiroz F. G., et al., Liquid-liquid phase separation drives skin barrier formation. Science 367, eeax9554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada S., Wirtz D., Coulombe P. A., Pairwise assembly determines the intrinsic potential for self-organization and mechanical properties of keratin filaments. Mol. Biol. Cell 13, 382–391 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L., Yamada S., Wirtz D., Coulombe P. A., A ‘hot-spot’ mutation alters the mechanical properties of keratin filament networks. Nat. Cell Biol. 3, 503–506 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Matsui T., et al., SASPase regulates stratum corneum hydration through profilaggrin-to-filaggrin processing. EMBO Mol. Med. 3, 320–333 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiokawa D., Tanuma S., Characterization of human DNase I family endonucleases and activation of DNase γ during apoptosis. Biochemistry 40, 143–152 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Eckhart L., Fischer H., Barken K. B., Tolker-Nielsen T., Tschachler E., DNase1L2 suppresses biofilm formation by Pseudomonas aeruginosa and Staphylococcus aureus. Br. J. Dermatol. 156, 1342–1345 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Fischer H., Buchberger M., Napirei M., Tschachler E., Eckhart L., Inactivation of DNase1L2 and DNase2 in keratinocytes suppresses DNA degradation during epidermal cornification and results in constitutive parakeratosis. Sci. Rep. 7, 6433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]