Abstract
Despite the impressive number of interlocked molecules described in the literature over the past 30 years, only a few stereoselective syntheses of mechanically chiral rotaxanes have been reported so far. In this study, we present the first diastereoselective synthesis of mechanically planar chiral [1]rotaxanes, that has been achieved using the active template Cu-mediated alkyne–azide cycloaddition reaction. This synthetic method has been applied to the preparation of a [1]rotaxane bearing a labile stopper that can then be substituted without disruption of the mechanical bond. This approach paves the way for the synthesis of a wide variety of mechanically planar chiral [1]rotaxanes, hence allowing the study of the properties and potential applications of this class of interlocked molecular architectures.
The first diastereoselective synthesis of mechanically planar chiral [1]rotaxanes has been achieved using the active template Cu-mediated alkyne–azide cycloaddition reaction.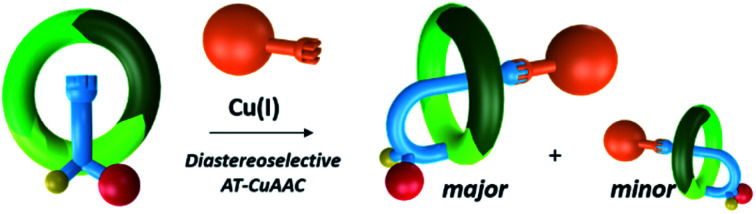
Introduction
Since Jean–Pierre Sauvage described the first templated synthesis of a [2]catenane in 1983,1 many approaches have been developed providing access to a wide diversity of rotaxanes and molecular knots.2 In contrast, stereoselective methods enabling the preparation of mechanically chiral interlocked molecules still remain sporadic. Initially, the enantiomers of some mechanically planar chiral (MPC) [1], [2] and [3]rotaxanes3 were isolated from racemic mixtures by means of preparative chiral HPLC.4 It was not until 2014 that Goldup and co-workers published the first strategy allowing the synthesis of MPC [2]rotaxanes without the need of chiral separation techniques.5 Using simple flash column chromatography, they separated the two diastereomers of a [2]rotaxane including a rotationally unsymmetrical macrocycle and an axle bearing a covalent chiral auxillary. The substitution of the latter by an achiral bulky entity then yielded each MPC [2]rotaxane enantiomer separately. A few years later, the same research group achieved the diastereoselective synthesis of MPC [2]rotaxanes via the active template Cu-mediated alkyne–azide cycloaddition (AT-CuAAC)6 reaction.7 In this case, the coupling of an alkyne and a chiral azide within the cavity of a bipyridine macrocycle gave MPC [2]rotaxanes with high diastereoselectivity. Subsequent symmetrisation of the covalent chiral centre led to the corresponding [2]rotaxane with the mechanical bond as the sole stereogenic element. Very recently, Leigh and co-workers reported a single-step enantioselective synthesis of MPC [2]rotaxanes, via the metal-free active template5,8N-acylation of a bulky amine with an activated ester bearing a chiral leaving group.9 In the presence of a crown ether lacking rotational symmetry, the substitution reaction occurred preferentially through one of the faces of the macrocycle via a diastereoisomeric transition state, thereby generating the MPC [2]rotaxane in 50% ee.
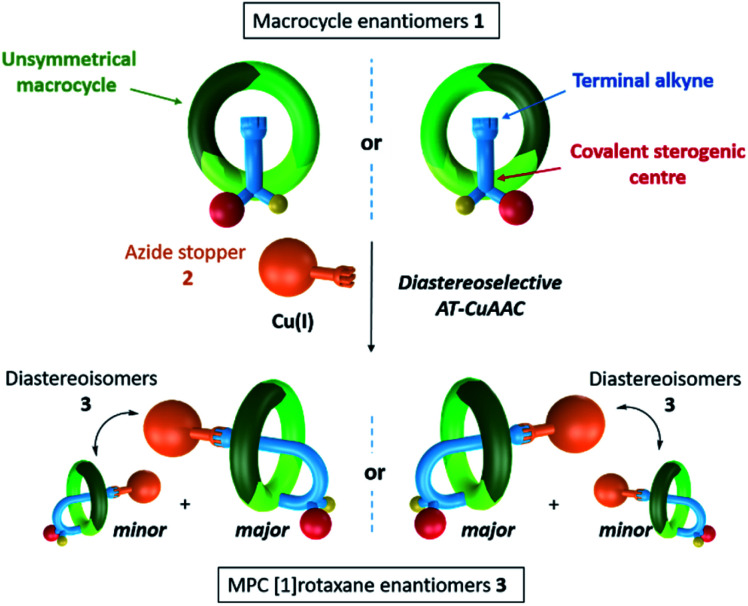
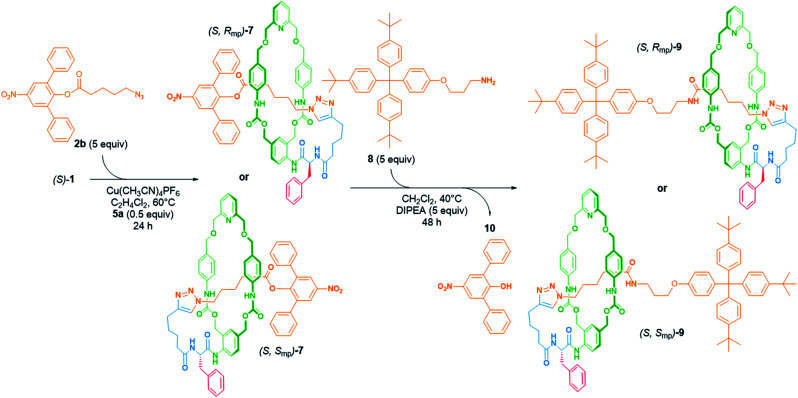
Herein, we present our study devoted to the development of the first diastereoselective synthesis of MPC [1]rotaxanes.10 Within this framework, we designed the rotationally unsymmetrical macrocycle 1 functionalised by a side chain including a covalent stereogenic centre and ended by a terminal alkyne (Fig. 1).
Fig. 1. Diastereoselective synthesis of mechanically planar chiral [1]rotaxanes via AT-CuAAC.
This latter reacted with azide stopper 2 through the AT-CuAAC to produce the corresponding molecular lasso 3 in a diastereoselective manner. In this approach, the mechanically planar chirality was directed by the absolute configuration of the stereogenic unit attached to the macrocycle, hence permitting the access to both [1]rotaxane enantiomers.
Results and discussion
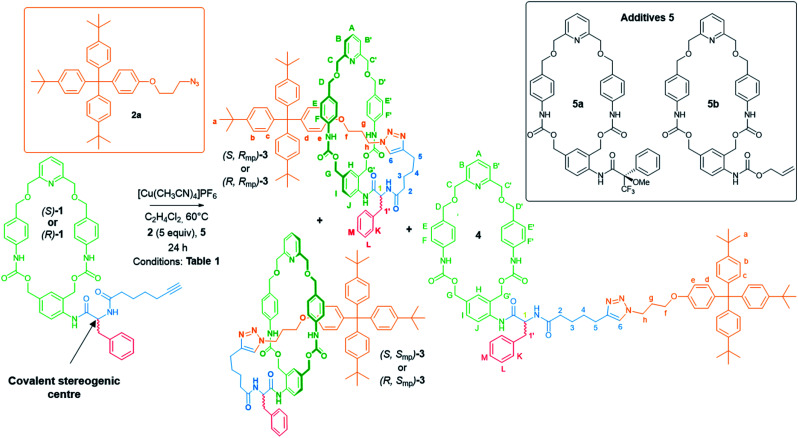
Prior to evaluating the potential of this synthetic strategy, we prepared the pyridine-containing macrocycle111 bearing a chiral phenylalanyl residue (Scheme 1, for the full synthesis of compound 1 see the ESI†). The first attempt to synthesize [1]rotaxane (S)-3 was carried out starting from the macrocycle (S)-1 in the presence of 5 equivalents of the azide 2a and 0.5 equivalent of [Cu(MeCN)4]PF6 (Table 1, entry 1). Under these conditions, we observed the formation of two new compounds, both corresponding to the empirical formula C87H96N8O9, which were identified as the [1]rotaxane (S)-3 and the corresponding non-interlocked isomer 4. The comparison of the 1H NMR spectra of (S)-3 and 4 (ref. 12) confirmed the interlocked molecular architecture of the [1]rotaxane and indicated the preferential localization of the macrocycle around the axle. As shown on Fig. 2, hydrogens atoms He, H6 and HA were shifted upfield (Δδ = 0.35, 1.20 and 0.35 ppm, respectively) suggesting π-stacking interactions between aromatic rings of the macrocycle and those of the axle. Furthermore, each benzylic protons HC,C′, HD,D′ and HG,G′ in the spectrum of [1]rotaxane (S)-3 exhibited two diastereotopic signals as the consequence of the presence of the axle within the cavity of the macrocycle. The aliphatic protons Hf–h were also significantly shifted upfield (Δδ = 1.08, 1.27 and 0.96 ppm, respectively) showing the preferential macrocycle location between the triazole and the stopper.
Scheme 1. Reaction conditions for the diastereoselective synthesis of [1]rotaxanes 3 from either the chiral macrocycles (S)-1 or (R)-1. Structure of the azides stopper 2a and the additives macrocycles 5.
Reaction conditions used for the diastereoselective synthesis of the [1]rotaxane 3.
| Entry | Cu(i) equiv. | Additive (equiv.) | 3/4 ratioc | Conversion (%) | Yield (%)d |
|---|---|---|---|---|---|
| 1 | 0.5 | None | 8/2 | 50 | 29 |
| 2 | 0.75 | None | 8/2 | 75 | 35 |
| 3 | 1 | None | 1/1 | 100 | 33 |
| 4 | 0.95 | None | 1/1 | 95 | 33 |
| 5 | 1a | None | 7/3 | 100 | 44 |
| 6 | 1 | 5ab (1) | 8/2 | 100 | 48 |
| 7 | 1 | 5ab (0.5) | 8/2 | 100 | 45 |
| 8 | 1 | 5ab (0.25) | 7/3 | 100 | 40 |
| 9 | 1 | 5bb (0.5) | 8/2 | 100 | 31 |
| 10e | 1 | 5ab (0.5) | 8/2 | 100 | 46 |
1 equivalent of Cu(i) was added portionwise as follow: 0.5, 0.25, 0.125 and 0.125 equivalent.
5 and 2a were added in the reaction mixture 30 min after 1 and the Cu(i) catalyst.
Determined by HPLC.
Isolated yield after purification by chromatography.
This reaction was carried out with the macrocycle (R)-1.
Fig. 2. 1H NMR spectra of (a) the [1]rotaxane (S)-3 and (b) the corresponding non-interlocked isomer 4.
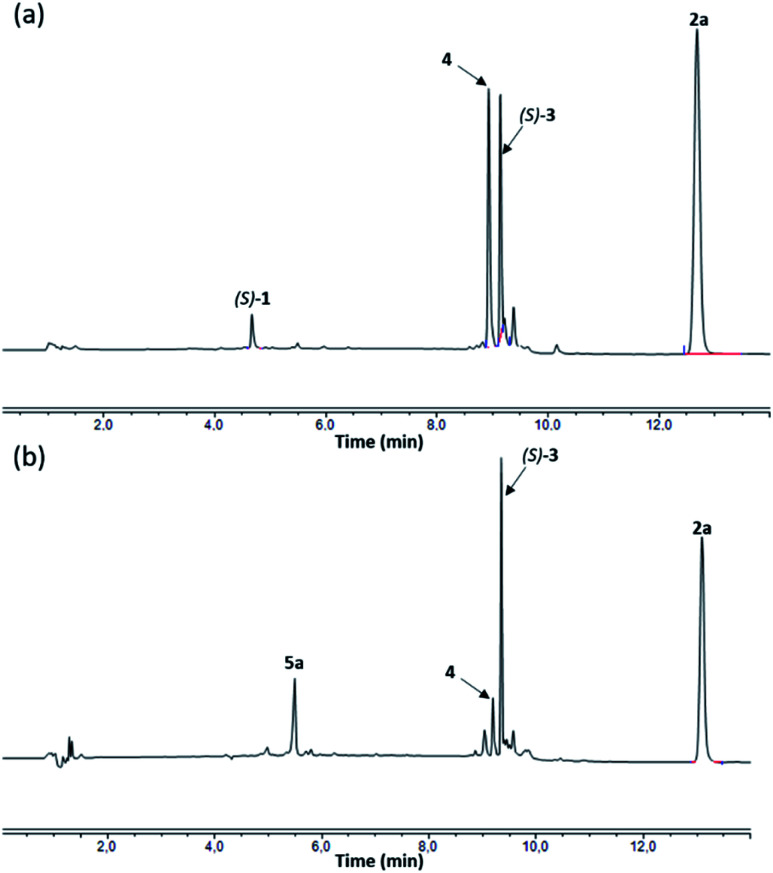
Under the reaction conditions described above (Table 1, entry 1), only 50% conversion of the starting macrocycle (S)-1 was observed. Furthermore, increasing the reaction time to 48 hours did not lead to additional consumption of the macrocycle (S)-1. Thus, we decided to pursue our investigations by enhancing the quantity of the Cu(i) catalyst to 0.75 equivalent (Table 1, entry 2). In this case, the conversion rate reached nearly 75% and the [1]rotaxane (S)-3 was isolated in 35% yield. Interestingly, such an increase in the amount of the catalyst did not induce a variation of the 3/4 ratio that was still in favour of the interlocked isomer (8/2, Table 1, entries 1 and 2). On the other hand, when the reaction was conducted with 1 equivalent of Cu(i) near complete conversion was observed, while the 3/4 ratio dropped to 1/1 (Table 1, entry 3). We first hypothesized that this loss of selectivity in disfavour of the interlocked molecule 3 was due to the presence of a slight excess of Cu(i) that could competitively catalyse the formation of the non-interlocked isomer 4.6 To avoid such an issue, we then carried out the reaction with only 0.95 equivalent of [Cu(MeCN)4]PF6 (Table 1, entry 4). After 24 hours under these conditions, the HPLC analysis of the crude showed incomplete conversion of the macrocycle (S)-1 (circa 95%), which was consistent with the amount of Cu(i) introduced in the reaction mixture, and a 3/4 ratio of 1/1 (Fig. 3a). Thus, in contrast to our hypothesis, this result indicated that the selectivity for the [1]rotaxane 3versus the isomer 4 decreased even with a substoichiometric quantity of the copper catalyst.
Fig. 3. HPLC traces of the crude mixture after 24 h of reaction. (a) Reaction conditions corresponding to entry 4, Table 1; (b) reaction conditions corresponding to entry 7, Table 1.
This first series of experiments was highly informative regarding the mechanism of formation of the [1]rotaxane (S)-3 and its non-interlocked analogue 4. Indeed, the conversion rate of macrocycle (S)-1 was directly correlated to the amount of Cu(i) introduced in the reaction mixture. This suggested that once coordinated to the endotopic pyridine of (S)-1, the metal catalyst cannot escape from the cavity of the macrocycle until its removal at the end of the reaction using an appropriate washing procedure (see the ESI†).
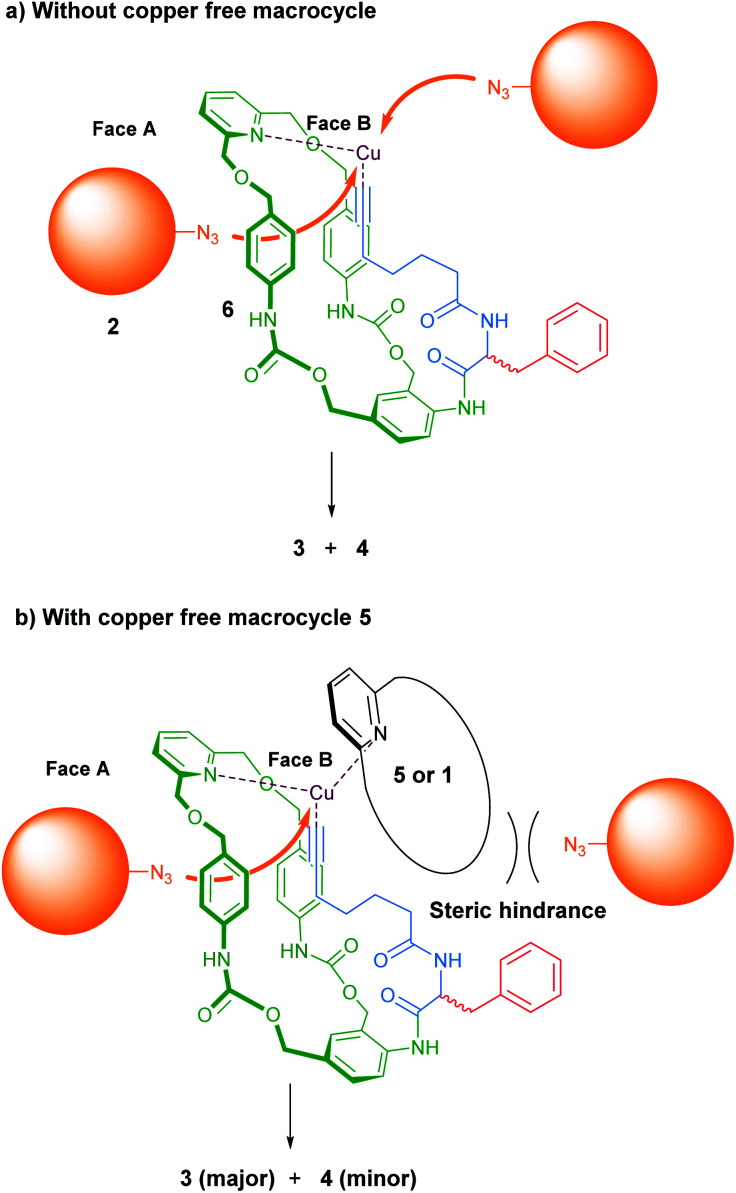
Under these circumstances, we postulated that (S)-3 and 4 arose from a same copper acetylide intermediate such as 6, in which both the metal and the side chain are positioned preferentially on the same face of (S)-1 (Fig. 4a). Thus, when the azide stopper 2 approaches the Cu(i) catalyst by the opposite face of the acetylide, the [1]rotaxane (S)-3 is formed, whereas the approach via the same face leads to the non-interlocked isomer 4 (face A and B respectively, Fig. 4a).
Fig. 4. Schematic representation of copper acetylide intermediates leading to the formation of 3 and 4.
We were also intrigued by the loss of selectivity for the [1]rotaxane (S)-3 when the reaction was carried out with 1 equivalent of Cu(i) (3/4 = 1/1 versus 8/2 with either 0.5 or 0.75 equivalent of Cu(i)). To explain this outcome, we suspected a potential role of the copper-free macrocycle present in the reaction mixture of experiments where substoichiometric amount of the catalyst was used. For investigating this hypothesis, we first performed an experiment in which 1 equivalent of Cu(i) was added portionwise in the reaction medium (Table 1, entry 6). In this case, each portion of the catalyst was introduced only once the previous one has accomplished its catalytic cycle and the reaction stopped (HPLC monitoring). In this manner, we were able to maintain some amount of copper-free macrocycle (S)-1 in the mixture until the addition of the last portion of Cu(i). As a result, the reaction went to completion with a 3/4 ratio of 7/3 and the [1]rotaxane (S)-3 was isolated in 44% yield.
We next conducted experiments with 1 equivalent of Cu(i) in the presence of the alkyne-deprived macrocycle 5a (Table 1, entries 6–8). Under these conditions, complete conversion of (S)-1 was observed leading to a 3/4 ratio in favour of the [1]rotaxane (S)-3 (8/2 and 7/3, with 0.5 to 1 and 0.25 equivalent of 5a, respectively), while macrocycle 5a was fully recovered.
Altogether, these experiments demonstrated that the presence of a copper-free macrocycle in the reaction mixture favoured the formation of the [1]rotaxane (S)-3vs. the non-interlocked isomer 4. In order to explain this outcome, we hypothesized that endotopic pyridine-containing macrocycles like 5 could reversibly bind the copper of the intermediate 6, thereby generating a steric hindrance on the face by which the azide 2 approaches for producing compound 4 (Fig. 4b). The same kind of intermediate could be formed with the macrocycle (S)-1 in cases where the reaction was conducted with substoichiometric quantity of Cu(i). This hypothesis is supported by the higher 3/4 ratio recorded when 1 equivalent of the copper catalyst has been added portionwise compared to its introduction all at once (Table 1, entries 1 and 4).
In the course of these experiments (Table 1, entries 1–8), only one diastereoisomer of the [1]rotaxane (S)-3 was isolated, suggesting that the reaction proceeded in a diastereoselective manner. For instance, when the reaction was conducted for 24 hours with 0.5 equivalent of the macrocycle 5a (Table 1, entry 7), the HPLC chromatogram of the crude mixture showed the presence of a major peak with a retention time of 9.36 minutes corresponding to one diastereoisomer of (S)-3 (Fig. 3b). Some other products, with retention times comprised between 8.5 and 10.0 minutes, were also formed. However, with the exception of the non-interlocked isomer 4 (r.t.: 9.19 min), none of these derivatives were produced in sufficient amount to be identified after purification by chromatography. These results were consistent with the 1H NMR spectrum of the crude mixture which demonstrated that compounds (S)-3 and 4 were the two main products of the reaction (see the ESI†). Therefore, while we cannot exclude that small amount of the other diastereoisomer of (S)-3 has been formed, these results demonstrated the diastereoselectivity of the AT-CuAAC reaction. However, in spite of numerous attempts, we did not succeed in producing a single-crystal in order to determine the relative configuration of the major diastereoisomer (S)-3 (Smp or Rmp,13Scheme 1).
It is also worth mentioning that the chiral centre of 5a did not affect the stereoselectivity of the reaction. Indeed, similar results were recorded when the reaction was undertaken using the achiral macrocycle 5b (Scheme 2), leading once again to the same major diastereoisomer of (S)-3 (Table 1, entry 9).
Scheme 2. Synthetic pathway for the diastereoselective preparation of [1]rotaxanes (S)-7 and (S)-9.
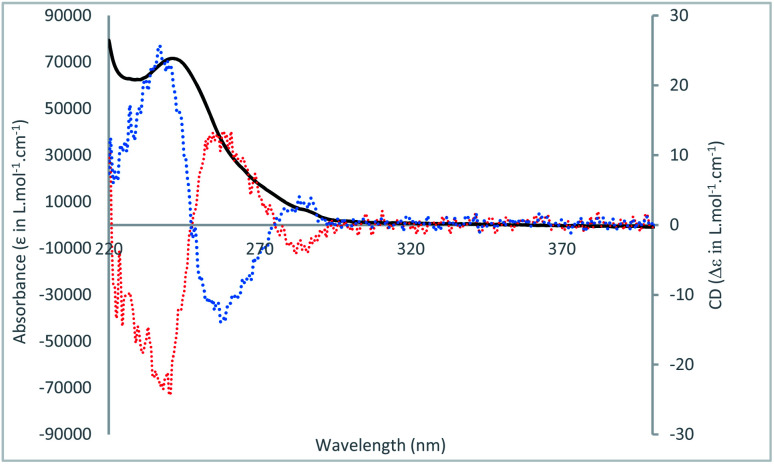
We next investigated the AT-CuAAC reaction with the mirror-image macrocycle (R)-1 (Table 1, entry 10). Under these conditions, we isolated a [1]rotaxane (46%) presenting identical 1H and 13C NMR spectra than the ones obtained using the macrocycle (S)-1 as the starting material. These results provided evidence that macrocycles (R)-1 and (S)-1 led to the formation of two enantiomers of [1]rotaxane 3. The enantiomeric nature of the [1]rotaxanes (S)-3 and (R)-3 was confirmed by circular dichroism analysis of the two MPC [1]rotaxanes that revealed mirror-image Cotton effects (Fig. 5).
Fig. 5. CD spectra of (S, Smp or S, Rmp)-3 (red dashed) and (R, Smp or R, Rmp)-3 (blue dashed). Absorbance spectrum (black). Measured in CH2Cl2 (C = 10−4 M) at 20 °C.
Thus, this study demonstrated that the absolute configuration of the stereogenic centre attached to the macrocycle directed the planar chirality of the resulting [1]rotaxane, thereby allowing the stereoselective synthesis of two enantiomers.
These results in hand, we decided to continue our investigations by extending the scope of this approach to the stereoselective preparation of other molecular lassos. For this purpose, we applied the reaction conditions described previously with the macrocycle (S)-1 and the azide stopper 2b (Scheme 2). In this case, the AT-CuAAC reaction led to the formation of the [1]rotaxane (S)-7 in 26% yield after purification by flash column chromatography. Furthermore, only one diastereoisomer was isolated from the crude mixture highlighting that the structure of the azide stopper has not affected the stereoselectivity of the reaction.
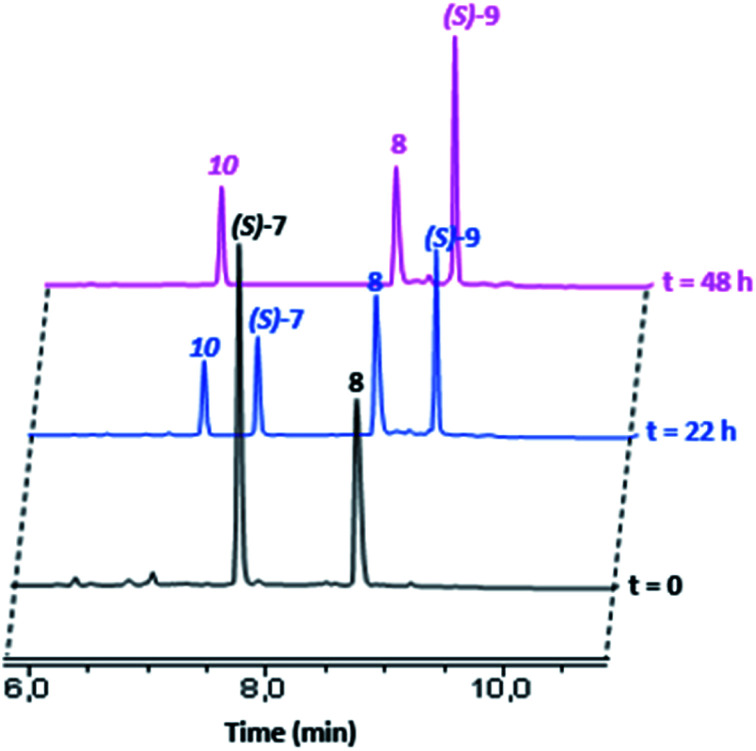
As the [1]rotaxane (S)-7 includes a labile bulky 2,6-diphenyl-4-nitrophenyl stopper,9h we then envisioned to use it as a molecular platform for the versatile access to various [1]rotaxanes. Thus, to evaluate such a synthetic strategy, we studied the nucleophile substitution reaction between (S)-7 and the primary amine 8 in CH2Cl2 at 40 °C (Scheme 2). The evolution of the reaction mixture was monitored by HPLC (Fig. 6).
Fig. 6. Reaction between (S)-7 and 8 in CH2Cl2 at 40 °C followed over the time by HPLC at t = 0 (black), t = 22 h (blue) and t = 48 h (magenta). Retention time: (S)-7 (7.97 min), 8 (8.76 min), (S)-9 (9.28 min) and 10 (7.32 min).
The chromatograms showed the disappearance of (S)-7 over the time and the emergence of two new peaks at 7.32 and 9.28 minutes which correspond to the nitrophenol 10 and the [1]rotaxane (S)-9, respectively. After 48 h under these conditions, complete consumption of lasso (S)-7 to (S)-9 was observed, without any detectable disruption of the mechanical bond. At the end of the reaction, the [1]rotaxane (S)-9 was purified by flash column chromatography and its interlocked structure confirmed by comparing its 1H NMR spectrum with that of the non-interlocked isomer (r.t.: 9.04 min, see the ESI†).
Conclusions
This study demonstrates that a covalent stereogenic centre attached closely to a macrocycle lacking rotational symmetry directs the diastereoselective synthesis of MPC [1]rotaxanes via the AT-CuAAC reaction. While the relative configuration of the main diastereoisomer still remains to be determined, the inversion of the absolute configuration of the chiral centre allows the stereoselective preparation of the mirror-image MPC [1]rotaxane. Since this method can be applied to the stereoselective synthesis of a [1]rotaxane bearing a labile stopper that can be substituted without disruption of the mechanical bond, it provides a versatile access to a diverse range of chiral molecular lassos. Thus, we believe this study constitutes a valuable starting point to explore the properties of MPC [1]rotaxanes as well as their potential applications.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
NP and AB thank the “Ministère de l’enseignement supérieur, de la recherché et de l’innovation” for financial support.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc05369d
Notes and references
- Dietrich C. O. Sauvage J.-P. Kintzinger J.-P. Tetrahedron Lett. 1983;24:5095. doi: 10.1016/S0040-4039(00)94050-4. [DOI] [Google Scholar]
- (a) Bruns C. J. and Stoddart J. F., The Nature of the Mechanical Bond: From Molecules to Machines, Wiley, 2016 [Google Scholar]; (b) Coutrot F. Waelès P. Angew. Chem., Int. Ed. 2020 doi: 10.1002/anie.202007496. [DOI] [Google Scholar]; (c) Gauthier M. Denis M. Goldup S. M. Nat. Rev. Chem. 2017;1:61. doi: 10.1038/s41570-017-0061. [DOI] [Google Scholar]; (d) Fielden S. D. P. Leigh D. A. Woltering S. L. Angew. Chem., Int. Ed. 2017;56:11166. doi: 10.1002/anie.201702531. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lewis J. E. M. Beer P. D. Loeb S. J. Goldup S. M. Chem. Soc. Rev. 2017;9:2577. doi: 10.1039/C7CS00199A. [DOI] [PubMed] [Google Scholar]; (f) Gil-Ramirez G. Leigh D. A. Stephens A. J. Angew. Chem., Int. Ed. 2015;54:6110. doi: 10.1002/anie.201411619. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Beves J. E. Blight B. A. Campbell C. J. Leigh D. A. McBurney R. T. Angew. Chem., Int. Ed. 2011;40:9260. doi: 10.1002/anie.201007963. [DOI] [PubMed] [Google Scholar]; (h) Crowley J. D. Goldup S. M. Lee A. L. Leigh D. A. McBurney R. T. Chem. Soc. Rev. 2009;6:1530. doi: 10.1039/B804243H. [DOI] [PubMed] [Google Scholar]
- Jamieson E. M. G. Modicom F. Goldup S. M. Chem. Soc. Rev. 2018;47:5266. doi: 10.1039/C8CS00097B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Yamamoto C. Okamoto Y. Schmidt T. Jäger R. Vögtle F. J. Am. Chem. Soc. 1997;119:10547. doi: 10.1021/ja971764q. [DOI] [Google Scholar]; (b) Reuter C. Mohry A. Sobanski A. Vögtle F. Chem.–Eur. J. 2000;6:1674. doi: 10.1002/(SICI)1521-3765(20000502)6:9<1674::AID-CHEM1674>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]; (c) Reuter C. Seel C. Nieger M. Vögtle F. Helv. Chim. Acta. 2000;83:630. doi: 10.1002/(SICI)1522-2675(20000315)83:3<630::AID-HLCA630>3.0.CO;2-1. [DOI] [Google Scholar]; (d) Kameta N. Hiratani K. Nagawa Y. Chem. Commun. 2004:466. doi: 10.1039/B314744D. [DOI] [PubMed] [Google Scholar]; (e) Makita Y. Kihara N. Nakakoji N. Takata T. Inagaki S. Yamamoto C. Okamoto Y. Chem. Lett. 2007;36:162. doi: 10.1246/cl.2007.162. [DOI] [Google Scholar]; (f) Ishiwari F. Nakazono K. Koyama Y. Takata T. Angew. Chem., Int. Ed. 2017;56:14858. doi: 10.1002/anie.201707926. [DOI] [PubMed] [Google Scholar]; (g) Gaedke M. Witte F. Anhäuser J. Hupatz H. Schröder H. V. Valkonen A. Rissanen K. Lützen A. Paulus B. Schalley C. A. Chem. Sci. 2019;10:10003. doi: 10.1039/c9sc03694f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h1) Tian C. Fielden S. D. P. Pérez-Saavedra B. Vitorica-Yrezabal I. J. Leigh D. A. J. Am. Chem. Soc. 2020;142:9803. doi: 10.1021/jacs.0c03447. [DOI] [PMC free article] [PubMed] [Google Scholar]; ; for examples of enantioseparation by HPLC of co-conformationally MPC rotaxanes, see: ; (i) Schmieder R. Hübner G. Seel C. Vögtle F. Angew. Chem., Int. Ed. 1999;38:3528. doi: 10.1002/(SICI)1521-3773(19991203)38:23<3528::AID-ANIE3528>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]; (j) Mochizuki Y. Ikeyatsu K. Mutoh Y. Hosoya S. Saito S. Org. Lett. 2017;19:4347. doi: 10.1021/acs.orglett.7b02043. [DOI] [PubMed] [Google Scholar]
- Bordoli R. J. Goldup S. M. J. Am. Chem. Soc. 2014;136:4817. doi: 10.1021/ja412715m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Aucagne V. Hänni K. D. Leigh D. A. Lusby P. J. Walker D. B. J. Am. Chem. Soc. 2006;128:2186. doi: 10.1021/ja056903f. [DOI] [PubMed] [Google Scholar]; (b) Aucagne V. Berná J. Crowley J. D. Goldup S. M. Hänni K. D. Leigh D. A. Lusby P. J. Ronaldson V. E. Slawin A. M. Z. Viterisi A. Walker D. B. J. Am. Chem. Soc. 2007;129:11950. doi: 10.1021/ja073513f. [DOI] [PubMed] [Google Scholar]
- Jinks M. A. de Juan A. Denis M. Fletcher C. J. Galli M. Jamieson E. M. G. Modicom F. Zhang Z. Goldup S. M. Angew. Chem., Int. Ed. 2018;57:14806. doi: 10.1002/anie.201808990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) De Bo G. Dolphijn G. McTernan C. T. Leigh D. A. J. Am. Chem. Soc. 2017;139:8455. doi: 10.1021/jacs.7b05640. [DOI] [PubMed] [Google Scholar]; (b) Fielden S. D. P. Leigh D. A. Mcternan C. T. Pérez-Saavedra B. Vitorica-Yrezabal I. J. J. Am. Chem. Soc. 2018;140:6049. doi: 10.1021/jacs.8b03394. [DOI] [PubMed] [Google Scholar]; (c) Tian C. Fielden S. D. P. Whitehead G. F. S. Vitorica-Yrezabal I. J. Leigh D. A. Nat. Commun. 2020;11:744. doi: 10.1038/s41467-020-14576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of grafting reactions involving rotaxanes with labile ester stoppers, see ref. 5 and: ; (a) Zehnder D. W. Smithrud D. B. Org. Lett. 2001;16:2485. doi: 10.1021/ol016107o. [DOI] [PubMed] [Google Scholar]; (b) Smukste I. Smithrud D. B. J. Org. Chem. 2003;68:2547. doi: 10.1021/jo026530q. [DOI] [PubMed] [Google Scholar]; (c) Smukste I. House B. E. Smithrud D. B. J. Org. Chem. 2003;68:2559. doi: 10.1021/jo026522+. [DOI] [PubMed] [Google Scholar]; (d) Dvornikovs V. House B. E. Kaetzel M. Dedman J. R. Smithrud D. B. J. Am. Chem. Soc. 2003;125:8290. doi: 10.1021/ja034918c. [DOI] [PubMed] [Google Scholar]; (e) Hannam S. Lacy S. M. Leigh D. A. Saiz C. G. Slawin A. M. Z. Stitchell S. G. Angew. Chem., Int. Ed. 2004;116:3260. doi: 10.1002/anie.200353606. [DOI] [PubMed] [Google Scholar]; (f) Kihara N. Motoda S. Yokozawa T. Takata T. Org. Lett. 2005;7:1199. doi: 10.1021/ol047639i. [DOI] [PubMed] [Google Scholar]; (g) Bao X. Isaacsohn I. Drew A. F. Smithrud D. B. J. Org. Chem. 2007;72:3988. doi: 10.1021/jo0623641. [DOI] [PubMed] [Google Scholar]; (h) Fernandes A. Viterisi A. Coutrot F. Potok S. Leigh D. A. Aucagne V. Papot S. Angew. Chem., Int. Ed. 2009;48:6443. doi: 10.1002/anie.200903215. [DOI] [PubMed] [Google Scholar]; (i) Chao S. Romuald C. Fournel-Marotte K. Clavel C. Coutrot F. Angew. Chem., Int. Ed. 2014;53:6914. doi: 10.1002/anie.201403765. [DOI] [PubMed] [Google Scholar]; (j) Waelès P. Clavel C. Fournel-Marotte K. Coutrot F. Chem. Sci. 2015;6:4828. doi: 10.1039/C5SC01722J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Legigan T. Riss-Yaw B. Clavel C. Coutrot F. Chem.–Eur. J. 2016;22:8835. doi: 10.1002/chem.201601286. [DOI] [PubMed] [Google Scholar]; (l) Riss-Yaw B. Morin J. Clavel C. Coutrot F. Molecules. 2017;22:2017. doi: 10.3390/molecules22112017. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Riss-Yaw B. Clavel C. Laurent P. Coutrot F. Chem. Commun. 2017;53:10874. doi: 10.1039/C7CC06598A. [DOI] [PubMed] [Google Scholar]; (n) Nierengarten I. Meichsner E. Holler M. Pieper P. Deschenaux R. Delavaux-Nicot B. Nierengarten J. F. Chem.–Eur. J. 2018;24:169. doi: 10.1002/chem.201703997. [DOI] [PubMed] [Google Scholar]; (o) Riss-Yaw B. Clavel C. Laurent P. Waelès P. Coutrot F. Chem.–Eur. J. 2018;24:13659. doi: 10.1002/chem.201802831. [DOI] [PubMed] [Google Scholar]
- For a review on the synthesis of [1]rotaxanes see: Coutrot F., in Advances in Atom and Single Molecule Machines, Single Molecular Machines and Motors, ed. C. Joachim and G. Rapenne, Springer International Publishing, Switzerland, 2015, p. 35 [Google Scholar]
- Barat R. Legigan T. Tranoy-Opalinski I. Renoux B. Péraudeau E. Clarhaut J. Poinot P. Fernandes A. E. Aucagne V. Leigh D. A. Papot S. Chem. Sci. 2015;6:2608. doi: 10.1039/C5SC00648A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The synthesis of the corresponding non-interlocked molecule 4 was done in parallel using conditions preventing any intramacrocyclic pre-organization of components (see ESI†)
- For the assignment of absolute stereochemistry of MPC rotaxanes see ref. 3. For the assignment of the relative configuration of [1]rotaxanes 3 see the ESI.†
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.