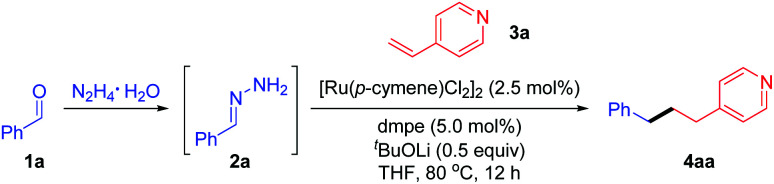
Optimization of reaction conditionsa.
| ||
|---|---|---|
| Entry | Variation from “standard conditions” | 4aab (%) |
| 1 | No change | 97 (95) |
| 2 | No [Ru(p-cymene)Cl2]2 | Trace |
| 3 | No base | Trace |
| 4 | Ru(PPh3)3Cl2 used instead | 96 |
| 5 | Ru(PPh3)3Cl2 used instead | 96 |
| 6 | [RuCl(p-cymene)(dppe)]Cl used instead | 71 |
| 7 | Cp*Ru(cod)Cl used instead | 24 |
| 8 | Ru(Cp)(PPh3)2Cl used instead | 14 |
| 9 | Ni(cod)2 used instead | N.D. |
| 10 | [Pd(allyl)Cl]2 used instead | N.D. |
| 11 | Dppe instead of dmpe | 96 |
| 12 | Dppb instead of dmpe | 84 |
| 13 | Dppf instead of dmpe | 66 |
| 14 | KOH instead of tBuOLi | 92 |
| 15 | K3PO4 instead of tBuOLi | 66 |
| 16 | K2CO3 instead of tBuOLi | 12 |
| 17 | DBU instead of tBuOLi | 10 |
Reaction conditions: benzaldehyde 1a (0.4 mmol), hydrazine (0.48 mmol), para-vinyl pyridine 3a (0.2 mmol), [Ru(p-cymene)Cl2]2 (2.5 mol%), ligand (5 mol%) and base (0.1 mmol) in THF (0.5 mL) at 80 °C for 12 h under N2 unless otherwise noted. Note that phenyl hydrazone 2a was generated in situ from benzaldehyde and hydrazine without isolation.
The yield of 4aa was determined by 1H NMR using mesitylene as an internal standard and based on 3a (isolated yield of 4aa in parentheses). N.D. = not detected.
