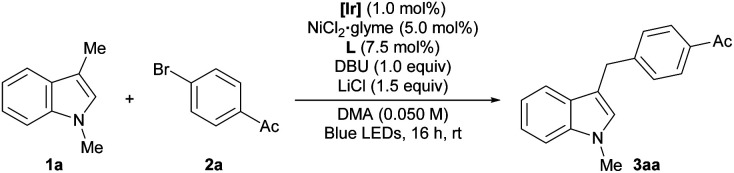
Optimization of reaction conditionsa.
| ||
|---|---|---|
| Entry | Conditions | Yield (%) |
| 1 | As shown | 83 (76b/73c) |
| 2 | No photocatalyst | N.D. |
| 3 | No light | N.D. |
| 4 | No NiCl2·glyme | N.D. |
| 5 | No DBU | 10 |
| 6 | No LiCl | 54 |
| 7 | With TEMPO (3.0 equiv.) | N.D. |
| 8 | ArCl instead of ArBr | 76 |
| 9 | ArI instead of ArBr | 16 |
| 10d | ArOTf instead of ArBr | 36 |
Reaction conditions: 1a (0.15 mmol), 2a (0.10 mmol), [Ir] (1.0 mol%), NiCl2·glyme (5.0 mol%), L (7.5 mol%), DBU (0.10 mmol), LiCl (0.15 mmol), and DMA (0.050 M) irradiated with 34 W blue LEDs. Yields were determined by 1H NMR analysis using 1,1,2,2-tetrachloroethane as an internal standard.
The reaction was set up using standard Schlenk technique on the benchtop.
The reaction was carried out under ambient conditions.
Without LiCl. [Ir] = Ir(dFCF3ppy)2(dtbbpy)PF6. dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine. L = 1,10-phenanthroline. DMA = N,N-dimethylacetamide. TEMPO = (2,2,6,6-tetramethylpiperidin-1-yl)oxyl. N.D. = not detected.
