Benzylic C(sp3)–H arylation of indoles by photoredox/nickel dual catalysisa.
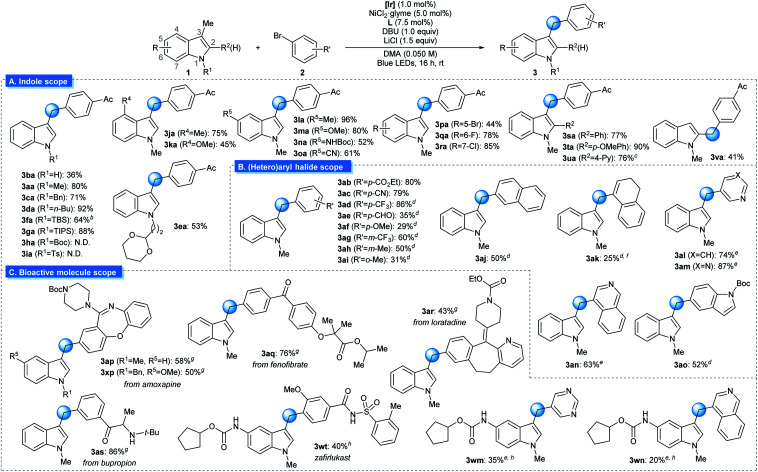
|
Reaction conditions: 1 (0.30 mmol), 2 (0.20 mmol), [Ir] (1.0 mol%), NiCl2·glyme (5.0 mol%), L (7.5 mol%), DBU (0.20 mmol), LiCl (0.30 mmol), and DMA (0.050 M) irradiated with 34 W blue LEDs. All yields are isolated yields.
18% of the desilylated product was obtained during the course of the reaction.
The reaction was performed with 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) instead of [Ir] for 23 h.
4,4′-dimethoxy-2,2′-bipyridine instead of L.
The reaction was conducted at 53 °C.
The corresponding vinyl triflate was used as substrate.
NiCl2·glyme (10.0 mol%), 4,4′-dimethoxy-2,2′-bipyridine (15.0 mol%), DMA (0.033 M), and ArCl instead of ArBr.
NiCl2·glyme (10.0 mol%), L (15.0 mol%), and DMA (0.033 M). [Ir] = Ir(dFCF3ppy)2(dtbbpy)PF6. dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine. L = 1,10-phenanthroline. DMA = N,N-dimethylacetamide. N.D. = not detected.
