Optimization of the reaction conditionsa.
| |||
|---|---|---|---|
| Entry | Variation of standard conditions | Yieldb | D-Inc.c |
| 1 | None | 75% | 96% |
| 2 | With 1a-Nad | 75% | 77% |
| 3 | With 1a-Ke | 75% | 83% |
| 4 | 4-(4-Methoxyphenyl)butanoic acid and CsOHf | 57% | 60% |
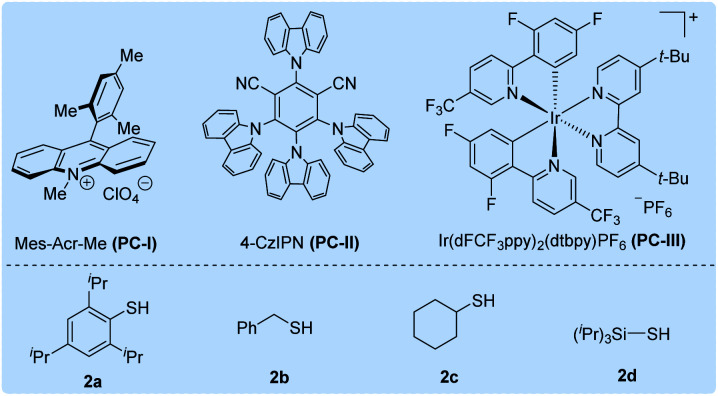
| 5 | PC-I instead of PC-III | 4% | — |
| 6 | PC-II instead of PC-III | 3% | — |
| 7 | 2b instead of 2a | Trace | — |
| 8 | 2c instead of 2a | 23% | 96% |
| 9 | 2d instead of 2a | 14% | 96% |
| 10 | Without PC-III or light | N.D. | — |
| 11 | Without 2a | 5% | — |
| |||
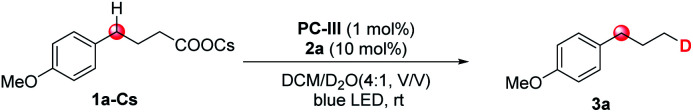
1a-Cs (0.2 mmol), PC-III (1 mol%), 2a (10 mol%), DCM/D2O (4 : 1, v/v; 2 mL, 110 eq. of D2O), 45 W blue LEDs, room temperature, 18 h.
Measured by GC using acetophenone as the internal standard due to the high volatility.
Deuterium incorporation was determined by HRMS-ESI.
Sodium 4-(4-methoxyphenyl)butanoate.
Potassium 4-(4-methoxyphenyl)butanoate.
CsOH (2.0 equiv.) as the base. DCM = dichloromethane, N.D. = not detected.
