Optimization of the reaction conditions for the decarboxylative radical addition to a glyoxylate-derived N-sulfinyl iminea.
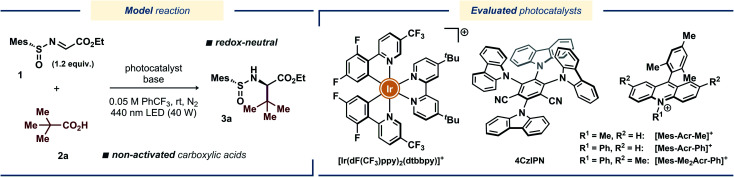
| |||||
|---|---|---|---|---|---|
| Entry | Photocatalyst | Base | Time | Yieldb | drb |
| 1c | [Ir(dF(CF3)ppy)2(dtbbpy)](PF6), 1 mol% | Cs2CO3, 0.2 equiv. | 20 min | — | — |
| 2 | [Ir(dF(CF3)ppy)2(dtbbpy)](PF6), 1 mol% | Cs2CO3, 0.2 equiv. | 20 min | 65% | 4 : 1 |
| 3 | 4CzIPN, 1 mol% | Cs2CO3, 0.2 equiv. | 20 min | — | — |
| 4 | [Mes-Acr-Me](BF4), 1 mol% | Cs2CO3, 0.2 equiv. | 20 min | 27% | >95 : 5 |
| 5 | [Mes-Acr-Me](BF4), 5 mol% | Cs2CO3, 0.2 equiv. | 20 min | 48% | >95 : 5 |
| 60 min | 66% | >95 : 5 | |||
| 6 | [Mes-Acr-Ph](BF4), 5 mol% | Cs2CO3, 0.2 equiv. | 60 min | 73% | >95 : 5 |
| 7 | [Mes-Me2Acr-Ph](BF4), 5 mol% | Cs2CO3, 0.2 equiv. | 60 min | 78% | >95 : 5 |
| 8 | [Mes-Me2Acr-Ph](BF4), 5 mol% | K3PO4, 0.2 equiv. | 60 min | 80% | >95 : 5 |
| 9 | [Mes-Me2Acr-Ph](BF4), 5 mol% | K2CO3, 0.2 equiv. | 60 min | 84% | >95 : 5 |
| 10 | [Mes-Me2Acr-Ph](BF4), 5 mol% | K2CO3, 0.05 equiv. | 20 min | <5% | — |
| 11 | [Mes-Me2Acr-Ph](BF4), 5 mol% | K2CO3, 0.5 equiv. | 60 min | 85% | >95 : 5 |
| 12d | [Mes-Me2Acr-Ph](BF4), 5 mol% | K2CO3, 0.5 equiv. | 60 min | 77% | >95 : 5 |
| 13 e | [Mes-Me 2 Acr-Ph](BF 4 ), 5 mol% | K 2 CO 3 , 0.5 equiv. | 60 min | 91% | >95 : 5 |
| 14f | [Mes-Me2Acr-Ph](BF4), 5 mol% | K2CO3, 0.5 equiv. | 60 min | 95% | >95 : 5 |
| 15 | As entry 13, but with tBu-sulfinyl imine 4 | 60 min | — | — | |
| 16 | As entry 13, but with p-Tol-sulfinyl imine 5 | 60 min | 50% | 7 : 1 | |
| Deviations from the conditions in entry 13 | |||||
| 17 | Under air | 60 min | 12% | >95 : 5 | |
| 18 | No photocatalyst | 60 min | — | — | |
| 19 | No light | 60 min | — | — | |
The reactions were performed on 0.1 mmol scale: stock solutions of pivalic acid 2 and the photocatalyst (each in 1 mL of the solvent) were mixed with N-sulfinyl imine 1 and the base under anhydrous conditions, and stirred under irradiation with 440 nm blue LED light at room temperature (for details, see the ESI).
Determined by 1H NMR of crude reaction mixture with 1,3,5-trimethoxybenzene as an internal standard.
0.1 M DMSO.
0.1 M PhCF3.
1.0 equiv. of N-sulfinyl imine 1 and 1.2 equiv. of pivalic acid 2.
1.0 equiv. of N-sulfinyl imine 1 and 1.5 equiv. of pivalic acid 2.
