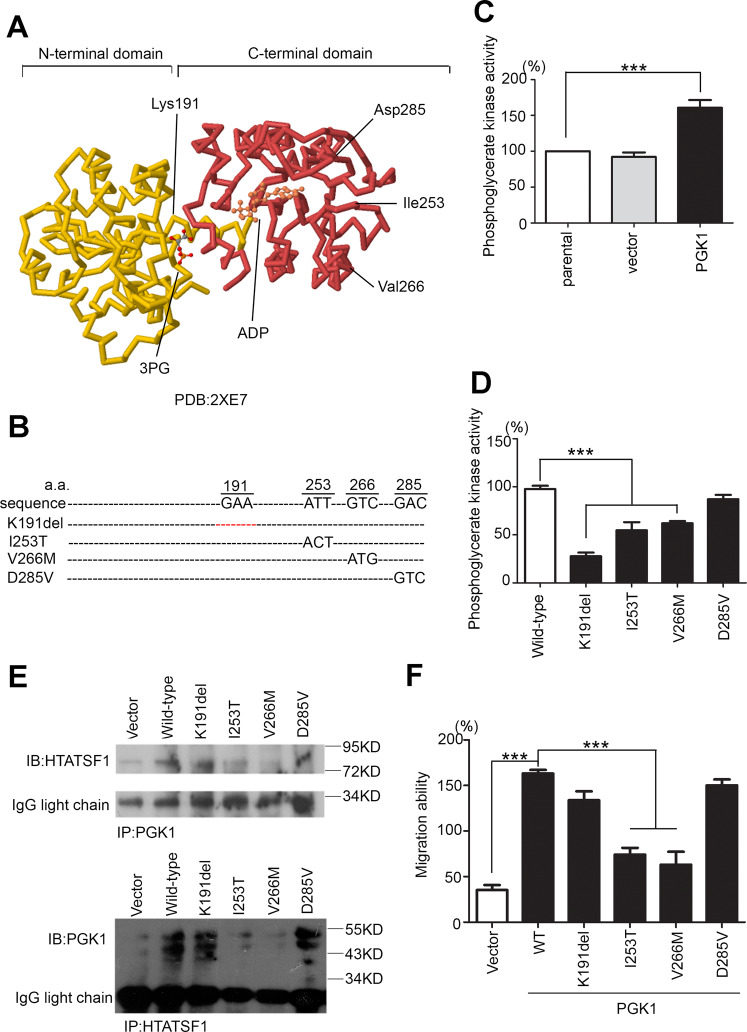
Fig. 6. The binding affinity of PGK1-HTATSF1 reflects the lung cancer migration ability.
A The crystal structure of PGK1 includes two domains and a 3-PG/ADP binding site (PDB ID:2XE7). B Sequences of the amino acids of the PGK1 wild-type gene, primer and several mutant forms that were designed. C Intracellular phosphoglycerate kinase activity of the PGK1 overexpression model. D Intracellular phosphoglycerate kinase activity in H1355 cells after the forced expression of exogenous wild-type or mutant PGK1 genes. E Pull-down assay for whole cell lysates derived from H1355 cells with the forced expression of exogenous wild-type (wt) or mutant PGK1 using beads followed by western blot analysis of the HTATSF1 and PGK1 proteins. F Cellular migration abilities of H1355 cells after the forced expression of exogenous wild-type or mutant PGK1. The symbol ***p < 0.001 in the nonparametric Mann–Whitney test.