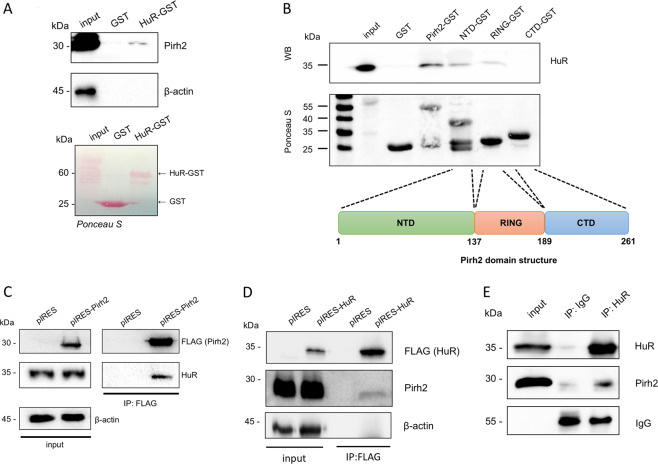
Fig. 2. Pirh2 binds to HuR in vitro and in cellulo.
A HuR-GST interacts with Pirh2. GST-pulldown assay with extracts from HEK293T, demonstrating the interaction of HuR-GST with Pirh2. A Ponceau-stained membrane. HuR-GST fusion protein and native GST protein are denoted with arrows (left); western blot analysis of Pirh2 binding to GST and HuR-GST (right). B Identification of the Pirh2 domains involved in HuR binding. WB analysis of GSTpull-down assay with purified GST-tagged proteins: GST, full-length Pirh2 (Pirh2-GST), the N-terminal domain of Pirh2 1–132 a.a. (NTD-GST), the RING domain of Pirh2 138–189 a.a. (RING-GST), and the C-terminal domain of Pirh2 190–261 a.a. (CTD-GST) (upper panel); the scheme demonstrating the Pirh2 domain structure (bottom panel). C Pirh2 interacts with HuR in cellulo. Western blot analysis of co-immunoprecipitation in HEK293T cells of ectopically expressed Pirh2-3×FLAG (pIRES-Pirh2) and endogenous HuR. An empty pIRES vector was used as negative control. D HuR interacts with Pirh2 in cellulo. Western blot analysis of co-immunoprecipitation in HeLa cells of ectopically expressed HuR-3×FLAG (pIRES-HuR) and endogenous Pirh2. An empty pIRES vector was used as negative control. E Co-immunoprecipitation of the endogenous HuR and Pirh2 proteins using anti-HuR monoclonal antibodies.