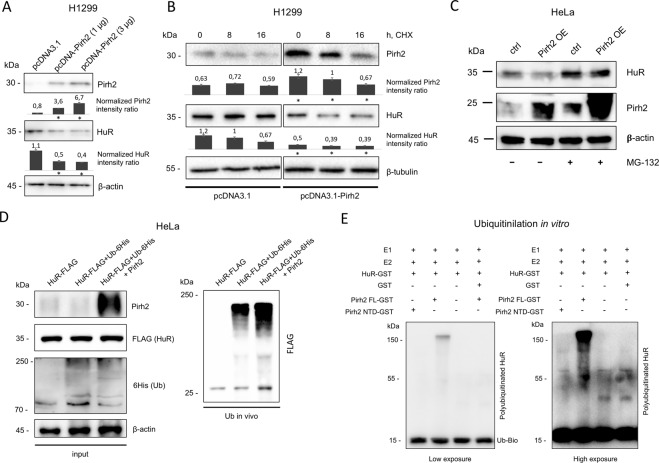
Fig. 3. Pirh2 downregulates HuR level via ubiquitination and targeting for the degradation.
A Pirh2 decreases HuR level in H1299 cells. Transfection of H1299 cells with different quantity of pcDNA3-Pirh2 plasmid proportionally decreases HuR level. Empty pcDNA3.1 was used as a control and to keep the total plasmid DNA amount 3 μg in each sample. The normalized intensity ratios were calculated as ratios between the signals of proteins analyzed and the corresponding actin bands on the basis of three measurements. Error bars indicate ± SD. *p ≤ 0.05 vs. empty vector transfection according to Student’s t-test. B Pirh2 shortens half-life time of HuR. Western blot analysis of HuR protein level in H1299 cells transfected with pcDNA-Pirh2 and pcDNA3.1 empty vector, and treated with 50 μM cycloheximide (CHX) for 8 and 16 h. The normalized intensity ratios were calculated as ratios between the signals of proteins analyzed and the corresponding actin bands on the basis of three measurements. Error bars indicate ±SD. *p ≤ 0.05 vs. empty vector transfection for the corresponding time points according to Student’s t-test. C WB analysis of HuR level in HeLa cells transiently transfected with pcDNA3-Pirh2 plasmid (Pirh2 OE) and pcDNA3.1 as a control (ctrl). The cells were treated with 10 μM MG132 for 16 h. D Pirh2 ubiquitinates HuR in cellulo. Western blot analysis of Ni-agarose-precipitated 6His-ubiquitinated HuR protein from HeLa cells transfected with pIRES-HuR (HuR-3×FLAG); pIRES-HuR and pcDNA-6His-Ubiquitin (Ub-6His); and pIRES-HuR, pcDNA-6His-Ubiquitin, and pcDNA-Pirh2 (Pirh2). pcDNA3.1 vector was used to keep the same amount of total plasmid DNA in each sample (right). Western blot analysis of Pirh2 and HuR-3×FLAG in inputs (left). E In vitro ubiquitinilation assay. Ubiquitin-activating enzyme (E1), UbcH5b (E2) and GST-tagged HuR (HuR-GST, substrate), and biotin-tagged ubiquitin were added in equal amounts in each portion of the reaction. GST, full-length Pirh2 (Pirh2 FL-GST), and the catalytically inactive N-terminal domain of Pirh2 1–132 a.a. (NTD-GST) were tested for their ability to ubiquitinate HuR. No E3 ligase control was also used. The WB analysis of the ubiquitinilation reaction was performed using anti-biotin antibodies. Two variants of exposure are shown for better illustration. The relative amounts of purified proteins used in the reaction are demonstrated in Supplementary Fig. S5.