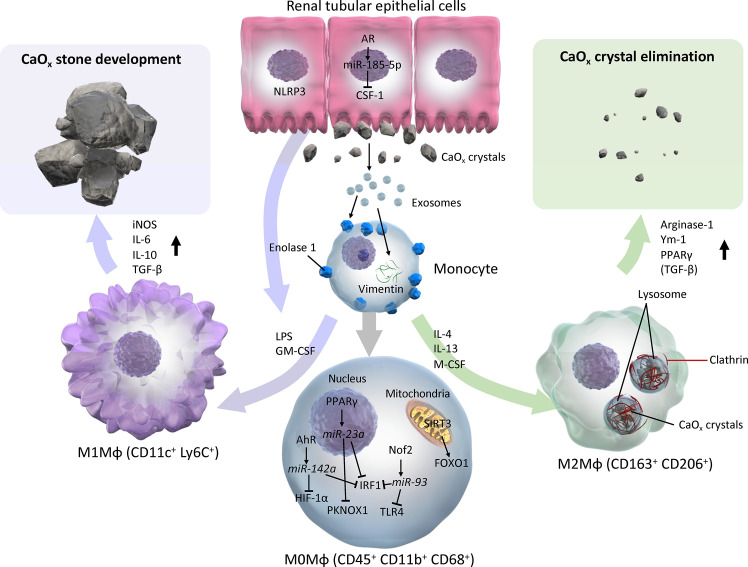
Figure 3.
Schema of evidence synthesized from current literature regarding roles of M1 and M2 macrophages in CaOx stone development. M1 or M2Mφs are usually differentiated from monocytes and M0 (with neutral polarization) Mφs via various cytokines/chemokines by direct and indirect influences from CaOx crystals. M0 Mφs autocrine mechanism shifts phenotypes toward CaOx crystal development via AhR-miR-142a-IRF1/HIF-1α, PPARγ-miR-23a-IRF1/PKNOX1, Nrf2-miR-93-IRF/TLR4, and SIRT3-FOXO1 axes. Monocytes that reflect exosomes secreted by monocytes/Mφs and renal tubular epithelial cells via enolase-1 and vimentin, then become activated and change into either M1 or M2Mφs. In contrast, paracrine involvement of renal tubular epithelial cells via NLRP3 and AR-miR-185-5p-CSF-1 is also an important factor in regulation of Mφ polarization. While M1Mφs facilitate CaOx stone development by promoting pro-inflammatory and oxidative stress molecules such as iNOS, IL-6, IL-10, and TGF-β, M2Mφs attenuate the development of CaOx crystals, and eliminate them by phagocytosis via lysosomes and clathrin mediation, and induces anti-inflammatory molecules including Arginase-1, Ym-1, and PPARγ. AhR, aryl hydrocarbon receptor; AR, androgen receptor; CSF-1, colony-stimulating factor-1; FOXO1, forkhead box O1; GM-CSF, granulocyte-macrophage colony-stimulating factor; HIF-1α, hypoxia-inducible factor-1 alpha; iNOS, inducible nitric oxide synthase; IRF1, interferon regulatory factor 1; LPS, lipopolysaccharide; Mφ, macrophage; M-CSF, macrophage colony-stimulating factor; NLRP3, nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain containing 3; PPARγ, peroxisome proliferator-activated receptor-gamma; SIRT3, Sirtuin 3; TLR4, toll-like receptor 4.