Abstract
This prospective, comparative, randomized, horizontal, and double-blind clinical study investigated the clinical efficacy of leucocyte-poor platelet-rich plasma (PRP, n=8) or allogeneic adipose-derived stem cells (ADSC, n=8) in dogs with bilateral degenerative hip joint disease (DHJD). Sixteen dogs were treated with two intra-articular injections of PRP or ADSCs, within a 30-day interval. The Canine Brief Pain Inventory (CBPI), the Helsinki Chronic Pain Index (HCPI), and Visual Analogue Scales for pain (VAS-pain) and locomotion (VAS-loc) were assessed by the dog owners. Analysis-of-gait using a force plate, response to palpation (VAS-palp), and the descriptive numerical scale for pain (DNS) were measured by a veterinarian. The assessments were performed before (baseline), 30 and 60 days after the first treatment. Data were analyzed using the unpaired t test, paired Wilcoxon test, Fisher’s exact test, and Mann-Whitney and Friedman tests (P<0.05). Compared with baseline HCPI, CBPI, VAS-pain, and VAS-palp scores reduced 41%, 52%, 51%, and 48% (P=0.0001–0.03) at 60 days in the ADSC group. In PRP-treated dogs, CBPI, VAS-loc, and DNS scores decreased by 43%, 43%, and 33% at 60 days, respectively (P=0.0003–0.011). Based on CBPI data, the rate of success at 60 days was 75% and 25% in the ADSC and PRP groups (P=0.13), respectively. Both therapies were apparently safe and effective to reduce chronic pain in dogs with bilateral DHJD during a 60-day period. However, a trend towards greater improvement was provided by the ADSC treatment.
Keywords: canine, hip dysplasia, mesenchymal stem cells, osteoarthritis, platelet-rich plasma
Osteoarthritis (OA) is a degenerative joint disease (DJD) commonly diagnosed in dogs suffering from hip dysplasia (HD), which is characterized by progressive loss of articular cartilage, resulting in pain, functional limitation, and decreased quality of life [16, 22]. Non-steroidal anti-inflammatory drugs alone or combined with other analgesics have been widely used for pain and inflammation relief associated with canine OA [6, 24, 28]. However, these medications are not adequately effective to prevent the deterioration of articular cartilage [1, 25] and when used for prolonged periods may induce potential adverse effects [24, 28].
Over the past two decades, there has been increasing interest in regenerative medicine approaches, including the use of mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP) for the treatment of DJD in both human and veterinary patients [4, 12, 13, 21, 23, 34, 39, 40].
MSCs may be obtained from various organic tissues, including bone marrow, synovium, periosteum, muscle, and the adipose tissue matrix [32, 43]. Adipose-derived stem cells (ADSCs) have been commonly used in clinical application due to their relatively easy accessibility and capability to differentiate into tissue cells with specialized function [31, 35]. The therapeutic benefits of stem cell-based therapies in osteoarthritic patients may be attributed to the ability to differentiate into chondrocytes and the paracrine effects. MSCs have been reported to be able to modulate inflammation and influence the local microenvironment, contributing to joint regeneration through production of bioactive elements and control of cytokine and growth factor secretion from endogenous cells [10, 32]. Recent clinical reports have demonstrated significant improvements in joint pain and limb function, without side effects in osteoarthritic dogs treated with MSCs [9, 13, 39].
PRP has been also considered an effective and safe therapy in animals affected by induced and naturally occurring OA [11, 12, 13, 40]. PRP consists of autologous plasma with a high concentration of platelets, rich in growth factors, which can promote chondrocyte proliferation and cartilage regeneration [11, 41]. Moreover, PRP plays an important role in OA-related inflammatory responses [5, 11, 44].
Although stem cells and PRP are substantially different, comparable therapeutic benefits have been reported from both intra-articular (IA) therapies [4, 13]. Cuervo et al. [13] reported that a single IA injection of ADSCs was as effective as PRP in reducing the clinical signs of OA in dogs suffering from hip dysplasia. Additionally, the combined IA administration of PRP and MSCs provided beneficial and synergic effects related to the synthesis and proliferation of chondrocytes and inhibition of inflammatory responses in surgical-induced OA in dogs [44].
Despite the increase in studies focused on the clinical efficacy of PRP and stem cells for osteoarthritic patients, the number of applications required to achieve a therapeutic effect has not been clarified. Data from a systematic review and meta-analysis in humans suggested that a single or multiple PRP injections provide comparable improvements in terms of joint pain and that multiple injections were more effective than a single injection for functional improvement [42]. With respect to MSC therapy, some human studies have demonstrated greater improvement following repeated injections [26, 34], while no significant differences between these two approaches were found in osteoarthritic horses [23]. To date, the majority of the published studies have focused on the effects of a single PRP or MSCs injection for the treatment of dogs with naturally occurring OA [12, 13, 17].
The aim of this study was to compare the effects of IA administration of repeated doses of ADSCs or leucocyte-poor PRP in terms of analgesia, limb function, quality of life, and adverse events over 60 days in client-owned dogs with bilateral DHJD. We hypothesized that both IA therapies would equally improve joint pain and limb function compared with pre-treatment values.
MATERIALS AND METHODS
Study design and ethics
This was a prospective, analytical, comparative, randomized, horizontal, and double-blind clinical study. The study was approved by the Institutional Ethical Committee for the Use of Animals in Research under protocol 35/2013, and written informed consent was obtained from all dog owners before recruitment to the study.
Study population
Sixteen client-owned dogs were enrolled in the study. Inclusion criteria were defined as the presence of clinical signs of pain for at least three months, orthopedic examination findings, and radiographic evidence consistent with bilateral degenerative hip joint disease (DHJD). Additionally, only dogs that had not received any kind of therapy, such as glucocorticoids, non-steroid anti-inflammatory drugs, nutraceuticals, phytotherapy, physiotherapy, or acupuncture for at least 30 days prior to enrolment at the study were included. Exclusion criteria were dogs with several systemic diseases, clinical signs consistent with other orthopedic diseases that induce pain or lameness, neurological diseases, and radiographic evidence of OA in other joints.
Radiographic evaluation
Lateral and ventrodorsal extended hip pelvic radiographs were taken to confirm the diagnosis and degree of DHJD. The images were evaluated by an experienced radiologist, and the degree of DHJD was scored using a scale ranging from 0 to 4 [15], where 0 =normal joint; 1 =radiographic evidence of instability; no degenerative change; 2 =mild degenerative change (occasional osteophytes); 3 =moderate degenerative change (osteophytes, subchondral sclerosis); and 4 =severe degenerative change (osteophytes, subchondral sclerosis, bone remodeling).
Collection, isolation, culture, and characterization of allogenic ADSCs (in vitro)
A 2-year-old client-owned Pit Bull female dog was selected as a donor of allogeneic MSC. Adipose tissue (4 g) was aseptically collected from the subcutaneous region around the tail under general anesthesia. The healthy status of the donor was evaluated by a complete blood count, serum chemistry profile, and serological testing for infectious diseases (Brucella canis, canine Herpesvirus, Erlichia canis, Leishmania sp., Leptospira sp., and Toxoplasma sp). The sample was processed as previously described [3]. The derived adipose tissue was washed with phosphate buffered saline (PBS), digested with 0.1% type I collagenase solution (Sigma Aldrich, St. Louis, MO, USA), filtrated, and washed twice by centrifugation for 5 min at 2,000 rpm in culture medium of 20% bovine fetal serum, combined with 77.8% low glucose DMEM, 1% penicillin, and 1.2% amphotericin B (Life Technologies, New York, NY, USA). The final pellet was homogenized in culture medium and distributed in sterile plastic culture flasks of 75 cm2 (37°C, 95% air and 5% CO2, 7 days) and the cell culture medium was replaced every four days. Cell replication was monitored through the level of confluence observed by microscopy. Once confluence was achieved (about 80%), trypsin (Sigma Aldrich) was used to disrupt adherence. ADSCs were characterized by their morphology, adherent quality, and osteogenic, chondrogenic, and adipogenic in vitro differentiation. Cell viability (fresh samples: 96.5% of whole cells and after cryopreservation 95.5% of whole cells) was investigated by flow cytometry (BD, Franklin Lakes, NJ, USA). The following surface monoclonal conjugated antibodies were used to evaluate the sample immunogenicity: CD 44 (Pre-cryopreservation: 88%, post-cryopreservation: 50.4%), CD34 (Pre-cryopreservation: 2.4%, post-cryopreservation: 2.8%), MHC class II (Pre-cryopreservation: 3.8%, post-cryopreservation: 3.4%), CD90 (Pre-cryopreservation: 99.4%, post-cryopreservation: 96.5%), and control (only cells) (Pre-cryopreservation: 1.9%, post-cryopreservation: 2.1%). The karyotype of ADSCs was studied in the second and fifth passages. All analysis showed normal characteristics of mitosis, without nucleus morphological changes.
Storage of ADSCs bank
The cells were cryopreserved in the second passage and diluted in cryoprotectant medium of 10% dimethyl sulfoxide and 90% fetal bovine serum with approximately 5 × 105 cells/cryotube. The tubes were submitted to slow cryopreservation and kept for 24 hr in an ultra-freezer at −80°C, cooled gradually by approximately 1°C per min, before being transferred to liquid nitrogen (−196°C) and maintained in this state until completion of the cultivation in the third passage.
Collection and processing of autologous leucocyte-poor PRP
Blood samples of 10 to 20 ml (for dogs weighing up to or more than 10 kg, respectively) were collected from the jugular vein of each dog to be treated with PRP. Blood was mixed with 3.2% sodium citrate. Samples were left without moving at 25° C for 30 min before the first centrifugation. PRP was prepared without the inclusion of the buffy coat. Mean baseline plasma platelet concentration was 2.7 × 105 platelets/µl and final mean PRP was 1 × 106 platelets/µl. Two centrifugations were carried out; the first was performed at 1,200 rpm for 10 min. The erythrocytes and the coat were discarded for the second centrifugation and the plasma was centrifuged at 1,600 rpm for 10 min. Approximately 80% of the supernatant plasma was discarded and the PRP was obtained. Immediately before IA infiltration PRP was activated using 10% calcium chloride [12, 40].
Intra-articular treatments
After sedation with intramuscular acepromazine (0.02 mg/kg; Acepran 0.2% Vetnil, São Paulo, Brazil) combined with tramadol (4 mg/kg; Tramadon Cristália, Itapira, Brazil) and subcutaneous local anesthesia with 1% lidocaine (Xyslestesin Cristália, Itapira, Brazil), the dogs were randomly assigned using an online software program (Research Randomizer, Computer software, http://www.randomizer.org/, Pennsylvania, PA, USA) to receive either IA PRP or 18 × 106 ADSCs bilaterally. In both groups the volume injected was 0.4 ml or 0.8 ml (for dogs weighing up to or more than 10 kg, respectively). The arthrocentesis was guided by ultrasound and the needle placement (22-g) was confirmed through aspiration of synovial fluid. The hip infiltration was performed at the beginning of the study and repeated after 30 days. Tramadol (3 mg/kg, q 12 hr) was administered orally for three days following IA injections.
Owner assessments
The dogs were evaluated by the owners using the Canine Brief Pain Inventory (CBPI), including the total pain scores (total CBPI score), pain severity scores (PSS), and pain interference scores (PIS) [8], as well as the Helsinki Chronic Pain Index (HCPI) [19], and a Visual Analogue Scale for pain (VAS-pain) [20] and locomotion (VAS-loc) [18]. Quality of life (QOL) was also evaluated using the following classification: bad=0, reasonable=1, good=2, very good=3, and excellent=4 [8]. Treatment success was defined as a reduction ≥1 in PIS and ≥2 in PSS [7]. The same person was responsible for assessing the dogs at each evaluation moment.
Orthopedic assessment
Prior to the beginning of the study, a complete orthopedic examination was performed by a single veterinarian, who measured the clinical signs using the Visual Analogue Scale for pain in response to palpation (VAS-palp) [20] and a descriptive numerical scale for pain (DNS) [36].
Gait analysis
A pressure platform system (WalkwayTM High Resolution HRV4, Tekscan Inc., Boston, MA, USA), calibrated according to the manufacturer’s recommendations, was used to measure the body mass distribution on pelvic and thoracic limbs. Dogs were guided to walk in a straight line with the conductor positioned on the right side. The speed was maintained between 0.9 and 1.1 m/sec and the acceleration between −0.15 and 0.15 m/sec [36]. Data acquisition and processing were performed using software (Walkway, version 7.0, Tekscan Inc.). A total of 5 valid trials were obtained for each dog for analysis.
Adverse effects
The dog owners were instructed to notify the occurrence of possible adverse events such as worsening pain, increased lameness, and temperature of affected joints during the study period.
Outcome measures
The primary outcome measures were the owner subjective measures and gait analysis. Secondary outcome measures were veterinary assessment and adverse effects. Data from gait analyses, owner and veterinary assessments were obtained prior to the beginning of the study (baseline), at 30 and 60 days post-treatment. Both owners and investigators were unaware of the treatment allocation.
Statistical analysis
A sample size of at least 8 dogs per group was estimated to achieve an 80% statistical power at an overall alpha level of 0.05, to detect a prevalence of treatment success of 80% and treatment failure of 20% in both treatment groups. The sample calculation was based on pilot data. The data were submitted to the Shapiro-Wilk normality test to identify the distribution. For the variables body weight, age, percentage improvement, and percentage body mass distribution of thoracic and pelvic limbs, the unpaired t test was used to compare the groups. A Mann-Whitney test was used to compare the owner and veterinarian scores between groups, whereas the differences over time within each group were compared using a Friedman test followed by the Dunn’s post-test. Except for QOL, the individual improvement was calculated by subtracting the scores obtained at 30 and 60 days from the baseline scores. The improvement in QOL was calculated by subtracting the baseline scores from the final scores (day 60). Treatment successes and failures were calculated using the Fisher’s exact test. The paired Wilcoxon test was used to compare the degree of DHJD between the right and left sides within groups, and also the sides affected in each group.
RESULTS
From twenty-five dogs screened for participation in the study, only sixteen met the inclusion criteria. Demographic data are presented in Table 1. The breeds included in this study were Labrador Retriever (6), Border Collie (2), Rottweiler (1), Schnauzer (1), Chow Chow (1), Belgian Shepherd (1), Saint Bernard (2), and Golden Retriever (2). There were no significant differences in body weight, age, and degree of DJHD between groups. There were no changes in the radiographic images before versus 60 days after treatment.
Table 1. Baseline characteristics of the study population.
| Paciente data | ADSC (n=8) | PRP (n=8) | P value |
|---|---|---|---|
| Body weight (kg) | 25.1 ± 12.7 | 24.0 ± 7.8 | 0.98 |
| Age (years) | 6.3 ± 3.9 | 4.6 ± 2.3 | 0.53 |
| Male/Female | 3/5 | 4/4 | 1.00 |
| OA degree/Right | 3.0 ± 0.7 | 3.0 ± 0.6 | 1.00 |
| OA degree/Left | 3.0 ± 0.7 | 3.0 ± 0.5 | 1.00 |
Values expressed as mean ± SD. ADSC, allogeneic adipose-derived stem cells; PRP, leucocyte-poor platelet-rich plasma.
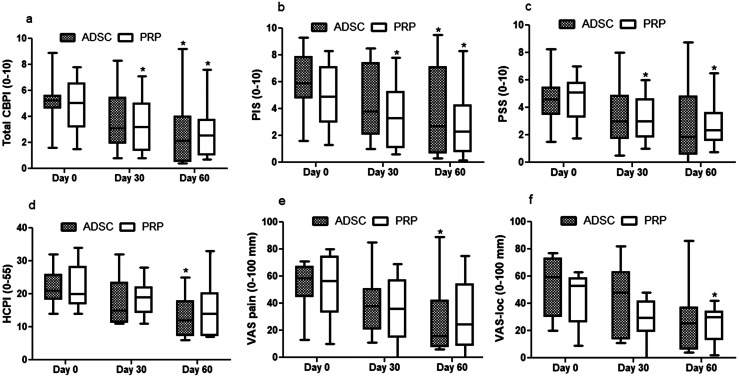
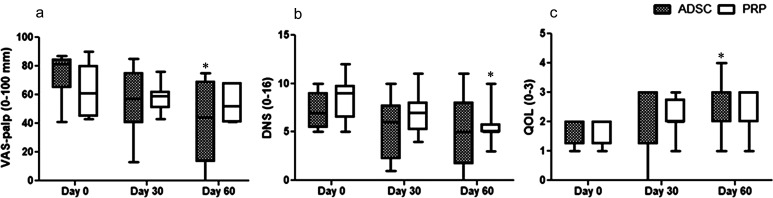
In ADSC-treated dogs, no significant changes were detected at 30 days in any outcome measures compared with baseline, while at 60 days total CBPI, PIS, PSS, HCPI, VAS-pain, and VAS-palp scores significantly reduced by 52%, 45%, 53%, 41%, 51%, and 48%, respectively (P=0.0001–0.03) (Figs. 1a–e and 2a). In PRP-treated dogs, total CBPI, PIS, and PSS scores significantly decreased by 33%, 33.5%, and 32% at 30 days and by 43%, 45%, and 40% at 60 days, respectively (P=0.0003–0.001) (Fig. 1a–c) while VAS-loc and DNS decreased 43% and 33%, respectively only at 60 days compared with pre-treatment values (P=0.011–0.024) (Figs. 1f and 2b).
Fig. 1.
Median (min–max) of the Canine Brief Pain Inventory (Total CBPI) (a), pain interference score (PIS) (b), pain severity score (PSS) (c), Helsinki Chronic Pain Index (HCPI) (d), Visual Analog Scale for pain (VAS-pain) (e) and Visual Analog Scale for locomotion (VAS-loc) (f) measured before (day 0) and up to 60 days after intra-articular injection of adipose-derived stem cells (ADSC, n=8) or platelet rich plasm (PRR, n=8) in dogs with bilateral degenerative hip joint disease. *Significantly different from day-0 (Friedman test, P<0.05).
Fig. 2.
Median (min–max) values of Visual Analog Scale for pain in response to palpation (VAS-palp) (a), descriptive numeric scale (DNS) (b) and quality of life (QOL) (c) scores measured before (day 0) and for up to 60 days after intra-articular injection of adipose-derived stem cells (ADSC, n=8) or platelet rich plasm (PRR, n=8) in dogs with bilateral degenerative hip joint disease. *Significantly different from day-0 (Friedman test, P<0.05).
Based on CBPI data, treatment was considered successful in 12.5% at 30 days in both groups, whereas at 60 days this rate improved to 25% and 75% in PRP and ADSC groups, respectively, but did not differ statistically between groups (P=0.13). The rate of success significantly improved between 30 and 60 days in the ADSC group (P=0.041) (Table 2). According to the impression of the dog owners, improvement in QOL was detected only in ADSC-treated dogs at 60 days (P=0.024) (Fig. 2c).
Table 2. Number and percentage (%) of treatment successes at 30 and 60 days after intra-articular injection of adipose-derived stem cells (ADSC) or platelet rich plasm (PRR) in dogs with bilateral degenerative hip joint disease.
| Success criteria | Treatment success |
P value | |
|---|---|---|---|
| PSS ≥ 2; PIS ≥ 1 | 30 days | 60 days | |
| ADSC (n=8) | 1 (12.5%) | 6 (75%)* | 0.041 |
| PRP (n=8) | 1 (12.5%) | 2 (25%) | 1 |
| P value | 1 | 0.13 | |
*Significant difference between 30 and 60 days (Fisher’s test, P<0.05). PSS, pain severity scores; PIS, pain interference scores.
The percentage of body mass distribution on thoracic and pelvic limbs did not differ within or between groups and between right and left pelvic limbs within each group (Table 3).
Table 3. Mean percentage (%) and standard deviation (SD) of body mass distribution on thoracic (TL) and pelvic limbs (PL) measured before (day 0) and up to 60 days after intra-articular injection of adipose-derived stem cells (ADSC) or platelet rich plasm (PRR) in dogs with bilateral degenerative hip joint disease.
| Group | Day 0 |
Day 30 |
Day 60 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TL |
PL |
TL |
PL |
TL |
PL |
|||||||
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | |
| ADSC (n=8) | 32.4 | 33.1 | 16.8 | 17.6 | 32.8 | 33.0 | 16.8 | 17.4 | 33.0 | 33.9 | 16.4 | 16.7 |
| SD | 2.0 | 1.8 | 1.6 | 2.2 | 2.5 | 2.2 | 2.3 | 2.0 | 2.2 | 2.2 | 2.2 | 1.9 |
| PRP (n=8) | 32.6 | 32.9 | 17.7 | 16.8 | 30.4 | 33.5 | 18.2 | 17.9 | 32.5 | 32.5 | 17.7 | 17.3 |
| SD | 5.4 | 3.3 | 3.5 | 4.7 | 4.6 | 6.0 | 1.3 | 3.4 | 3.5 | 1.7 | 1.2 | 3.7 |
No local or systemic adverse events were recorded during the 60-day period of the study.
DISCUSSION
This study showed that both therapies improved chronic pain behaviors associated with hip OA, which is consistent with previously reported findings in osteoarthritic dogs treated with PRP or ADSCs [12, 13, 17]. However, no evidence of clinical benefit was found in the gait analysis.
Although significant intergroup differences were not detected in any of the primary or secondary outcome measures, based on the owner and veterinary assessments, significant improvements were detected in 7 out of 9 of the outcome variables (total CBPI, PSS, PIS, HCPI, QOL, VAS-pain, VAS-palp) in the ADSC group and in only 4 out of 9 of the outcome variables (PSS, PIS, VAS-loc, DNS) in the PRP group. Moreover, the rate of treatment success at the end of the study was greater, but not statistically significant, in ADSC (75%) over PRP (25%). These findings suggest that MSC therapy was apparently more effective than PRP, which is not in agreement with a previous study that reported comparable effects in terms of subjective measures, including pain, functional limitation, and quality of life following a single IA PRP or ADSCs injection in dogs suffering from hip OA, except at 6 months, where greater improvement was observed in MSC-treated dogs [13]. The discrepancy between results may be attributed to the different methods used to quantify the clinical improvement and the definition of treatment response. In the previous study only the median differences in scores between groups were used to compare treatment responses, while in the present study, as well as this approach, the response to treatment was classified for each dog, as recommended by Brown et al. [7]. Using this method, it is possible to quantify the effectiveness of each treatment in an individual patient, whereas the comparison between median scores reflects the treatment response in a group of patients. When considering the median PSS and PIS scores, comparable improvements were identified in both groups, while only a modest improvement was detected in PRP-treated dogs when treatment success was defined as a reduction of at least 1 point in PIS and 2 points in PSS. This finding suggests that the great improvements observed in a small number of dogs may have masked the lack of treatment response in other dogs in the group. This was not unexpected, since large sample sizes have been recommended for clinical OA studies [6, 7, 24, 28]. The subjective outcome variables used in the current study to quantify treatment effects have been commonly reported in OA canine trials [29, 36]. Both CBPI and HCPI are validated reliable questionnaires to assess chronic pain in dogs [8, 19]. Although the VAS scale has not been properly validated, it is also an accepted method of chronic pain assessment in dogs [12, 13, 36].
The therapeutic potential may also be influenced by other factors such as the variability in PRP and stem cell preparation. In the current study, both substances were produced under controlled laboratory conditions, using commonly accepted methods for their preparation [17, 44]. In addition, PRP was prepared with a low concentration of leukocytes to prevent a risk of inflammatory cellular response, which has been associated with PRP preparations rich in leucocytes [14, 27]. Another important aspect is the source of the stem cells. Previous studies have demonstrated that bone marrow, synovial, and adipose mesenchymal stem cells demonstrate chondrogenic potential and may provide therapeutic benefits in osteoarthritic patients [32, 43]. In the current study, the decision on the source for ADSCs was based on the facility of extraction of adipose tissue and minimal risk of complications to the donors. Moreover, the choice for allogenic instead of autologous ADSCs was based on the shorter time required for cell isolation and expansion, lower cost of the procedure, and possibility of selecting healthy and young donors. Additionally, data from human and animal studies have demonstrated that the dosage of IA MSCs plays an important role in the efficacy and safety of treatment. In our protocol, we choose an intermediate dosage of ADSCs, based on previous studies that reported IA dosages from 10 × 106 to 30 × 106 of MSCs for osteoarthritic dogs [9, 13, 17, 44]. In agreement with prior clinical reports [9, 12, 13], no local or systemic adverse events were observed in this study. As transitory mild to moderate local joint pain has commonly been reported after IA injection [13, 40], to avoid this discomfort, tramadol was given for 3 days post-treatment in all the dogs.
Regarding the clinical benefits over time, a significant progressive response to treatment was detected only in the ADSC group, where the rate of success increased from 12.5% to 75%, which could be attributed either to the time required to obtain a positive response to treatment or to the second ADSCs injection, or both. Recent clinical human studies have shown superior beneficial effects following repeated IA stem-cell injections compared to a single injection for treatment of knee osteoarthritis [26, 34]. In dogs, several studies have concluded that MSCs are an effective approach to treat different affected joints [9, 13, 17, 39], however none of them have compared the treatment response following single or multiple injections.
In terms of objective outcome measures, biomechanical force platforms are considered sensitive methods to measure the impact of treatment on limb function [6, 28, 40]. Consistent with previously published data in osteoarthritic dogs [30, 36], no improvements were detected in gait analyses parameters in either of the treatments. This finding might be related to the joint assessed, since gait analysis in dogs with hip OA is complex, due to multifaceted changes in locomotion, which could confound kinetic measures [2, 36]. Recently, one study showed comparable kinetic data between dogs with clinical signs of HD and pain-free dogs with no HD [36]. Moreover, muscle atrophy has been identified in most dogs with bilateral hip OA [2, 12], which is an important factor that can impair the gait analysis. Thus, the 60-day period of evaluation may have been insufficient to detect improvements in kinetic variables, because biomechanical alterations are slow to take place.
The similarity between pre and post-treatment radiographic examinations was expected. Other OA studies have also found no evidence of radiographic changes after IA PRP or MSCs compared with baseline or placebo treatment [13, 33]. Although radiographic exams are commonly used to evaluate joint diseases, more specific methods such as magnetic resonance imaging and arthroscopy may be more appropriate to evaluate the cartilage repair process. In addition, a follow-up of at least 6 months could provide better understanding regarding the radiographic improvement or progression of DJD.
Potential limitations of this study include the small sample size and lack of a control group. In uncontrolled studies, the positive treatment responses may be overestimated, mainly with a relatively small number of subjects, as part of the improvement could be attributed to a placebo effect and not necessarily to an intervention.
In studies using client-owned dogs, the owner assessment has been recognized as a more sensitive instrument than veterinary assessment to quantify treatment response, because the daily behavior of the dog in its home environment appears to be superior to the periodic assessment in clinical settings [8]. However, changes in the home environment and owner bias may influence the outcome measures. A previous OA canine study reported a positive treatment response in 79.2% and 55.4% of dogs receiving ADSCs or placebo, respectively [17]. Moreover, small sample sizes have been associated with low statistical power, which could justify in part the lack of statistical changes between pre and post-treatment in the gait analyses in both groups. Furthermore, the current study did not investigate the combined IA administration of both therapies. In animal models, a synergistic effect has been reported following PRP plus ADSCs IA injection, leading to clinical therapeutic benefits in terms of cartilage regeneration and joint function [38, 44]. In dogs suffering from hip OA, significant improvements were detected in kinetic variables after IA coadministration of PRP and ADSCs [37, 41]. Additionally, the baseline characteristics of the study population are pivotal to understanding the individual treatment responses. Despite the apparent homogeneity between groups in terms of age, body weight, and degree of DHJD, it was impossible to classify the Norberg angle and the HD score, because the dog owners did not consent to the use of general anesthesia, impairing the ideal positioning for radiographic examination. Thus, it is possible that the severity of HD was underestimated for some patients, which could interfere in both subjective and objective outcome measures. The short-term assessment is another limitation of this study. A follow-up evaluation of at least 6 months has been considered a minimum standard to analyze the outcome measures of different treatments in osteoarthritic dogs [9, 39, 40]. Data from human and canine clinical trials have demonstrated that joint pain, mobility, and functional improvements after both PRP and MSC therapies ranged from 6 to 24 months [9, 12, 13, 37].
Both therapies were apparently safe and effective to reduce chronic pain behaviors in dogs with bilateral hip OA during the 60-day period. However, a trend towards greater improvement was provided by ADSCs. Additional larger, controlled, and long-term studies are needed to support these results and to clarify the true impact of multiple injections on the clinical benefit.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
The authors thank Marianne Camargo Dias, Danielle Jaqueta Barberini and Washington Kano for technical assistance.
REFERENCES
- 1.Aguiar G. C., Queiroz-Junior C. M., Sitta G. L., Amaral F. A., Teixeira M. M., Caliari M. V., Ferreira A. J.2016. Mefenamic acid decreases inflammation but not joint lesions in experimental osteoarthritis. Int. J. Exp. Pathol. 97: 438–446. doi: 10.1111/iep.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnoczky S. P., Torzilli P. A.1981. Biomechanical analysis of forces acting about the canine hip. Am. J. Vet. Res. 42: 1581–1585. [PubMed] [Google Scholar]
- 3.Barberini D. J., Freitas N. P., Magnoni M. S., Maia L., Listoni A. J., Heckler M. C., Sudano M. J., Golim M. A., da Cruz Landim-Alvarenga F., Amorim R. M.2014. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential. Stem Cell Res. Ther. 5: 25. doi: 10.1186/scrt414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bąkowski P., Kaszyński J., Wałecka J., Ciemniewska-Gorzela K., Bąkowska-Żywicka K., Piontek T.2020. Autologous adipose tissue injection versus platelet-rich plasma (PRP) injection in the treatment of knee osteoarthritis: a randomized, controlled study-study protocol. BMC Musculoskelet. Disord. 21: 314. doi: 10.1186/s12891-020-03345-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendinelli P., Matteucci E., Dogliotti G., Corsi M. M., Banfi G., Maroni P., Desiderio M. A.2010. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J. Cell. Physiol. 225: 757–766. doi: 10.1002/jcp.22274 [DOI] [PubMed] [Google Scholar]
- 6.Brown D. C., Boston R. C., Farrar J. T.2013. Comparison of force plate gait analysis and owner assessment of pain using the Canine Brief Pain Inventory in dogs with osteoarthritis. J. Vet. Intern. Med. 27: 22–30. doi: 10.1111/jvim.12004 [DOI] [PubMed] [Google Scholar]
- 7.Brown D. C., Bell M., Rhodes L.2013. Power of treatment success definitions when the Canine Brief Pain Inventory is used to evaluate carprofen treatment for the control of pain and inflammation in dogs with osteoarthritis. Am. J. Vet. Res. 74: 1467–1473. doi: 10.2460/ajvr.74.12.1467 [DOI] [PubMed] [Google Scholar]
- 8.Brown D. C., Boston R. C., Coyne J. C., Farrar J. T.2008. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J. Am. Vet. Med. Assoc. 233: 1278–1283. doi: 10.2460/javma.233.8.1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabon Q., Febre M., Gomez N., Cachon T., Pillard P., Carozzo C., Saulnier N., Robert C., Livet V., Rakic R., Plantier N., Saas P., Maddens S., Viguier E.2019. Long-term safety and efficacy of single or repeated intra-articular injection of allogeneic neonatal mesenchymal stromal cells for managing pain and lameness in moderate to severe canine osteoarthritis without anti-inflammatory pharmacological support: pilot clinical study. Front. Vet. Sci. 6: 10. doi: 10.3389/fvets.2019.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan A. I., Dennis J. E.2006. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98: 1076–1084. doi: 10.1002/jcb.20886 [DOI] [PubMed] [Google Scholar]
- 11.Chouhan D. K., Dhillon M. S., Patel S., Bansal T., Bhatia A., Kanwat H.2019. Multiple platelet-rich plasma injections versus single platelet-rich plasma injection in early osteoarthritis of the knee: an experimental study in a guinea pig model of early knee osteoarthritis. Am. J. Sports Med. 47: 2300–2307. doi: 10.1177/0363546519856605 [DOI] [PubMed] [Google Scholar]
- 12.Cuervo B., Rubio M., Chicharro D., Damiá E., Santana A., Carrillo J. M., Romero A. D., Vilar J. M., Cerón J. J., Sopena J. J.2020. Objective comparison between platelet rich plasma alone and in combination with physical therapy in dogs with osteoarthritis caused by hip dysplasia. Animals (Basel) 10: 175. doi: 10.3390/ani10020175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuervo B., Rubio M., Sopena J., Dominguez J. M., Vilar J., Morales M., Cugat R., Carrillo J. M.2014. Hip osteoarthritis in dogs: a randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. Int. J. Mol. Sci. 15: 13437–13460. doi: 10.3390/ijms150813437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragoo J. L., Braun H. J., Durham J. L., Ridley B. A., Odegaard J. I., Luong R., Arnoczky S. P.2012. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am. J. Sports Med. 40: 1274–1281. doi: 10.1177/0363546512442334 [DOI] [PubMed] [Google Scholar]
- 15.Flückiger M.2008. Scoring radiographs for canine Hip Dysplasia−The big three organisations in the world. EJCAP 17: 135–140. [Google Scholar]
- 16.Gold R. M., Gregor T. P., Huck J. L., McKelvie P. J., Smith G. K.2009. Effects of osteoarthritis on radiographic measures of laxity and congruence in hip joints of Labrador Retrievers. J. Am. Vet. Med. Assoc. 234: 1549–1554. doi: 10.2460/javma.234.12.1549 [DOI] [PubMed] [Google Scholar]
- 17.Harman R., Carlson K., Gaynor J., Gustafson S., Dhupa S., Clement K., Hoelzler M., McCarthy T., Schwartz P., Adams C.2016. A prospective, randomized, masked, and placebo-controlled efficacy study of intraarticular allogeneic adipose stem cells for the treatment of osteoarthritis in dogs. Front. Vet. Sci. 3: 81. doi: 10.3389/fvets.2016.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hielm-Björkman A. K., Kuusela E., Liman A., Markkola A., Saarto E., Huttunen P., Leppäluoto J., Tulamo R. M., Raekallio M.2003. Evaluation of methods for assessment of pain associated with chronic osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 222: 1552–1558. doi: 10.2460/javma.2003.222.1552 [DOI] [PubMed] [Google Scholar]
- 19.Hielm-Björkman A. K., Rita H., Tulamo R. M.2009. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am. J. Vet. Res. 70: 727–734. doi: 10.2460/ajvr.70.6.727 [DOI] [PubMed] [Google Scholar]
- 20.Holton L. L., Scott E. M., Nolan A. M., Reid J., Welsh E., Flaherty D.1998. Comparison of three methods used for assessment of pain in dogs. J. Am. Vet. Med. Assoc. 212: 61–66. [PubMed] [Google Scholar]
- 21.Kavadar G., Demircioglu D. T., Celik M. Y., Emre T. Y.2015. Effectiveness of platelet-rich plasma in the treatment of moderate knee osteoarthritis: a randomized prospective study. J. Phys. Ther. Sci. 27: 3863–3867. doi: 10.1589/jpts.27.3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkby K. A., Lewis D. D.2012. Canine hip dysplasia: reviewing the evidence for nonsurgical management. Vet. Surg. 41: 2–9. doi: 10.1111/j.1532-950X.2011.00928.x [DOI] [PubMed] [Google Scholar]
- 23.Magri C., Schramme M., Febre M., Cauvin E., Labadie F., Saulnier N., François I., Lechartier A., Aebischer D., Moncelet A. S., Maddens S.2019. Comparison of efficacy and safety of single versus repeated intra-articular injection of allogeneic neonatal mesenchymal stem cells for treatment of osteoarthritis of the metacarpophalangeal/metatarsophalangeal joint in horses: A clinical pilot study. PLoS One 14: e0221317. doi: 10.1371/journal.pone.0221317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malek S., Sample S. J., Schwartz Z., Nemke B., Jacobson P. B., Cozzi E. M., Schaefer S. L., Bleedorn J. A., Holzman G., Muir P.2012. Effect of analgesic therapy on clinical outcome measures in a randomized controlled trial using client-owned dogs with hip osteoarthritis. BMC Vet. Res. 8: 185. doi: 10.1186/1746-6148-8-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastbergen S. C., Jansen N. W., Bijlsma J. W., Lafeber F. P.2006. Differential direct effects of cyclo-oxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: an in vitro study. Arthritis Res. Ther. 8: R2. doi: 10.1186/ar1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matas J., Orrego M., Amenabar D., Infante C., Tapia-Limonchi R., Cadiz M. I., Alcayaga-Miranda F., González P. L., Muse E., Khoury M., Figueroa F. E., Espinoza F.2019. Umbilical cord-derived mesenchymal stromal cells (mscs) for knee osteoarthritis: repeated MSC dosing is superior to a single msc dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl. Med. 8: 215–224. doi: 10.1002/sctm.18-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarrel T. M., Minas T., Fortier L. A.2012. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J. Bone Joint Surg. Am. 94: e143, 1–8. doi: 10.2106/JBJS.L.00019 [DOI] [PubMed] [Google Scholar]
- 28.Moreau M., Dupuis J., Bonneau N. H., Desnoyers M.2003. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet. Rec. 152: 323–329. doi: 10.1136/vr.152.11.323 [DOI] [PubMed] [Google Scholar]
- 29.Nicácio G. M., Luna S. P. L., Cavaleti P., Cassu R. N.2019. Intra-articular botulinum toxin A (BoNT/A) for pain management in dogs with osteoarthritis secondary to hip dysplasia: A randomized controlled clinical trial. J. Vet. Med. Sci. 81: 411–417. doi: 10.1292/jvms.18-0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsen A., Johnson V., Webb T., Santangelo K. S., Dow S., Duerr F. M.2019. Evaluation of intravenously delivered allogeneic mesenchymal stem cells for treatment of elbow osteoarthritis in dogs: A Pilot Study. Vet. Comp. Orthop. Traumatol. 32: 173–181. doi: 10.1055/s-0039-1678547 [DOI] [PubMed] [Google Scholar]
- 31.Reich C. M., Raabe O., Wenisch S., Bridger P. S., Kramer M., Arnhold S.2012. Isolation, culture and chondrogenic differentiation of canine adipose tissue- and bone marrow-derived mesenchymal stem cells—a comparative study. Vet. Res. Commun. 36: 139–148. doi: 10.1007/s11259-012-9523-0 [DOI] [PubMed] [Google Scholar]
- 32.Sasaki A., Mizuno M., Mochizuki M., Sekiya I.2019. Mesenchymal stem cells for cartilage regeneration in dogs. World J. Stem Cells 11: 254–269. doi: 10.4252/wjsc.v11.i5.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva R. F., Carmona J. U., Rezende C. M.2013. Intra-articular injections of autologous platelet concentrates in dogs with surgical reparation of cranial cruciate ligament rupture: a pilot study. Vet. Comp. Orthop. Traumatol. 26: 285–290. doi: 10.3415/VCOT-12-06-0075 [DOI] [PubMed] [Google Scholar]
- 34.Song Y., Du H., Dai C., Zhang L., Li S., Hunter D. J., Lu L., Bao C.2018. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen. Med. 13: 295–307. doi: 10.2217/rme-2017-0152 [DOI] [PubMed] [Google Scholar]
- 35.Taguchi T., Borjesson D. L., Osmond C., Griffon D. J.2019. Influence of donor’s age on immunomodulatory properties of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 28: 1562–1571. doi: 10.1089/scd.2019.0118 [DOI] [PubMed] [Google Scholar]
- 36.Teixeira L. R., Luna S. P., Matsubara L. M., Cápua M. L., Santos B. P., Mesquita L. R., Faria L. G., Agostinho F. S., Hielm-Björkman A.2016. Owner assessment of chronic pain intensity and results of gait analysis of dogs with hip dysplasia treated with acupuncture. J. Am. Vet. Med. Assoc. 249: 1031–1039. doi: 10.2460/javma.249.9.1031 [DOI] [PubMed] [Google Scholar]
- 37.Upchurch D. A., Renberg W. C., Roush J. K., Milliken G. A., Weiss M. L.2016. Effects of administration of adipose-derived stromal vascular fraction and platelet-rich plasma to dogs with osteoarthritis of the hip joints. Am. J. Vet. Res. 77: 940–951. doi: 10.2460/ajvr.77.9.940 [DOI] [PubMed] [Google Scholar]
- 38.Van Pham P., Bui K. H., Ngo D. Q., Vu N. B., Truong N. H., Phan N. L., Le D. M., Duong T. D., Nguyen T. D., Le V. T., Phan N. K.2013. Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res. Ther. 4: 91. doi: 10.1186/scrt277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilar J. M., Batista M., Morales M., Santana A., Cuervo B., Rubio M., Cugat R., Sopena J., Carrillo J. M.2014. Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet. Res. 10: 143. doi: 10.1186/1746-6148-10-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vilar J. M., Manera M. E., Santana A., Spinella G., Rodriguez O., Rubio M., Carrillo J. M., Sopena J., Batista M.2018. Effect of leukocyte-reduced platelet-rich plasma on osteoarthritis caused by cranial cruciate ligament rupture: A canine gait analysis model. PLoS One 13: e0194752. doi: 10.1371/journal.pone.0194752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vilar J. M., Morales M., Santana A., Spinella G., Rubio M., Cuervo B., Cugat R., Carrillo J. M.2013. Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet. Res. 9: 131. doi: 10.1186/1746-6148-9-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilchez-Cavazos F., Millán-Alanís J. M., Blázquez-Saldaña J., Álvarez-Villalobos N., Peña-Martínez V. M., Acosta-Olivo C. A., Simental-Mendía M.2019. Comparison of the clinical effectiveness of single versus multiple injections of platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Orthop. J. Sports Med. 7: 2325967119887116. doi: 10.1177/2325967119887116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshimura H., Muneta T., Nimura A., Yokoyama A., Koga H., Sekiya I.2007. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 327: 449–462. doi: 10.1007/s00441-006-0308-z [DOI] [PubMed] [Google Scholar]
- 44.Yun S., Ku S. K., Kwon Y. S.2016. Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. J. Orthop. Surg. Res. 11: 9. doi: 10.1186/s13018-016-0342-9 [DOI] [PMC free article] [PubMed] [Google Scholar]