Abstract
Objective:
To determine the frequency of autoimmune antibody testing in an inpatient neurology setting and its influence on immunotherapy use on an inpatient neurology service.
Methods:
A retrospective descriptive cohort study of patients admitted to the neurology inpatient service at a large tertiary academic medical center who had autoimmune and/or paraneoplastic antibody testing performed between 10/1/2017 and 10/1/2018. Characteristics of patients’ initial clinical presentation, antibody testing results, test timing in relation to initiating immunotherapy, and final diagnosis using consensus criteria were extracted and analyzed. Case reports of patients with positive antibody panels are presented.
Results:
Of 1,604 patients, 50 patients (3.1%) had an antibody panel sent. Tests resulted after an average of 17 days (range 7-27). The most common clinical presenting symptom in those with a panel sent was encephalopathy. There were 5 (10%) positive serum panels and no positive CSF panels. Only one of these 5 patients had autoimmune encephalitis and was treated with immunotherapy. Of those with negative serum and CSF panels, 15 were treated acutely with empiric immunotherapy and the remainder with supportive care. Of those treated with immunotherapy, 14/15 (93%) were treated before the panel tests resulted. Four patients who had negative panels but were empirically treated met consensus criteria for an autoimmune-mediated neurologic process.
Conclusion:
Our study suggests that the results of antibody testing did not influence inpatient neurologists’ decision to treat with immunotherapy as most treatments began prior to final results being available.
Keywords: autoimmune diseases of the nervous system, neuroimmunology, neurohospitalist, spine disorders
Background and Purpose
Autoimmune and paraneoplastic antibody testing has advanced our understanding of previously indistinct neurologic presentations. Diagnosis and treatment of autoimmune encephalitis can in some cases reverse neurologic states including coma and rapidly progressive dementia. Appropriate testing may also detect malignancy earlier when intracellularly targeted antibodies are found, and more prompt diagnosis and treatment of the underlying cancer may lead to improved neurological outcomes. 1,2
Antibody panels have allowed physicians to order a number of diagnostic tests simultaneously and efficiently. Given that paraneoplastic antibodies are found in only approximately 60% of patients with central nervous system paraneoplastic neurologic syndromes, 3 a negative test result does not exclude the syndrome. 4 Additionally, recent works suggest a large proportion of antibody testing produces false positive results. 5,6 Together, these factors could create risks of both over and underdiagnosing these conditions. In the inpatient setting, clinicians may have varying familiarity with antibody testing and may have difficulty interpreting subtle details associated with some of the autoimmune panel results. Yet, antibody testing is important for guiding screening for malignancy and treatment decisions.
Prior work has included the antibody panel results from patients admitted to an inpatient service, however to our knowledge, there has been no report of testing this unique population of inpatients admitted to the neurology service. 5,7,8 Further, we did not find any reports about the role of testing on inpatient neurology management of patients.
In this study, we sought to determine the rates of antibody testing amongst patients admitted to our inpatient neurology service and to determine how testing for these antibodies framed real world inpatient treatment decisions. We secondarily identified the number of false and true positives amongst the cohort of individuals for whom these panels were sent. We present the case reports of patients with positive antibody results and discuss the complexity of interpreting these results.
Methods
Study Population
This is a descriptive cohort study and case series. The electronic medical records of patients admitted to the Neurology service at Brigham and Women’s Hospital between 10/1/2017 and 10/1/2018 (1,604 patients) were reviewed for paraneoplastic or autoimmune antibody testing in cerebrospinal fluid (CSF), serum or both. The terms “autoimmune” and “paraneoplastic” were electronically searched in each chart throughout all notes during inpatient admissions. Then, labs were reviewed to see if a panel was sent during inpatient admissions. Patients with a known autoimmune condition that were admitted to the inpatient neurology service were excluded in this study.
All charts were reviewed independently by 2 different clinical practitioners once. There were 2 cases that panels were sent but missed by 1 clinical practitioner on initial review. These 2 cases were included in the total number of panels sent. Demographic information and other case specifics were all entered by 1 individual. All CSF testing was performed through the Mayo Medical Laboratory. Serologic testing was all sent to Mayo as well. Additional serologic testing was sent to other sites. Fifty patients were identified as having testing performed. Amongst this group, multiple characteristics were recorded including age, sex, race and ethnicity, time from symptom onset, presenting symptoms, date of admission, date antibody testing was sent, date antibody testing returned, immunotherapy received while hospitalized, and date of immunotherapy received. Presenting symptom were recorded in a descriptive fashion and then grouped into the following: infectious cause, toxic/metabolic causes, limbic encephalitis, sarcoidosis, immune-mediated encephalitis, immune-mediated myelitis, immune-mediated peripheral neuropathy or radiculopathy, neurodegenerative, neoplastic, and other. Additionally a number of other tests and evaluations were recorded. Lumbar punctures were analyzed for white cell count, red cell count, protein, glucose and the presence of oligoclonal bands. MRI result were collected and categorized as abnormal due to non-specific changes, abnormal with T2 flair changes consistent with a possible autoimmune basis and normal MRI. MRIs were also categorized as abnormal or normal. EEGs were also collected and categorized as those with epileptic activity (either seizure or epileptiform discharges) or abnormal with slowing and normal. Additionally, follow-up information including ongoing immunotherapy, diagnosis, imaging and malignancy screening was recorded.
Analysis
We calculated the proportion of patients who had antibody panels sent in the serum, CSF and both. We calculated the percent of patients that were female. We calculated the proportion of patients with positive antibody tests. We determined whether treatments had been initiated prior to the results of the panels being available using dates that had been obtained with chart review. We calculated the mean time from test sent to result. We used consensus criteria for cases of autoimmune encephalitis 9 and myelitis 10 as the standard for diagnosing patients with these conditions. A false positive was defined as when an antibody test returned positive and the patient did not meet consensus criteria. A false negative was defined as when the antibody test returned negative but the consensus criteria indicated the diagnosis was consistent with an autoimmune cause. This study was conducted with the approval of the Institutional Review Board of the Partners Human Research Committee. A descriptive analysis is provided due to the small number of patients with panels sent.
Results
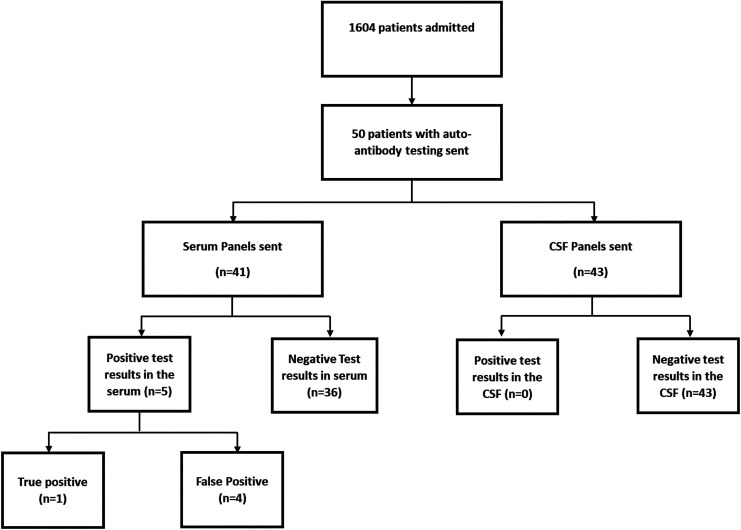
Of 1,604 patients admitted during the study period, 50 patients (3.1%) had an antibody panel sent from the CSF and/or serum (Figure 1). The mean age of patients with a panel sent was 60 years (range 20-88 years) and 28 (56%) of them were female.
Figure 1.
Flow chart of patient admitted and patients with auto-antibody testing of different types and respective outcomes.
Amongst these patients, there were 43 CSF and 41 serum panels sent. Both serum and CSF testing were performed in 19 patients (38%). Both paraneoplastic and autoimmune panels were sent in 4 patients. Tests resulted after an average of 17 days (range 7-27 days). No CSF panels returned a positive result.
The most common clinical presenting symptom in those with a panel sent was encephalopathy. Amongst all patients admitted during the year reviewed, 225 (14%) presented with encephalopathy. Amongst those that panels were sent on, 27 (54%) presented with encephalopathy.
Thirty eight patients with panels sent (76%) underwent an EEG. Of those patients most had some degree of abnormal slowing (19 patients, 38%) or epileptic activity (13 patients, 26%). The majority of patients 49/50 (98%) underwent lumbar puncture of which 32/50 (64%) were abnormal defined as having a protein greater than 45 mg/dL or CSF white blood cell count greater than 5 cells per uL. All 50 patients underwent MRI brain and/or spine of which 41 had some abnormality on imaging including T2 hyperintense lesions, enhancing lesions, and/or cerebral atrophy.
There were 5 positive serum panels. Only one of those 5 patients had a relevant autoimmune syndrome (as determined by consensus guidelines) and was treated with immunotherapy (Table 1).
Table 1.
Description of Patients with Positive Serum Panel Results.
| Case # | Antibody | Titer | Sex | Age | CSF | MRI abnormal | Final diagnosis | Immunotherapy and response |
|---|---|---|---|---|---|---|---|---|
| 1 | PQ-VGCC | 0.07mmol/L | F | 71 | No | Yes- scattered T2 changes | CNS Lymphoma | Improved with chemotherapy |
| 2 | NMDA | Positive at 1:10 dilution | F | 68 | No | No | Polypharmacy | Improved with reduction in polypharmacy |
| 3 | GAD65 | 0.04 mmol/L | F | 79 | No | Yes, non-specific white-matter changes | Non-Alzheimer’s Dementia | Improved with supportive care |
| 4 | GAD65 | 0.06 mmol/L | M | 45 | Yes | Yes- diffuse leukoencephalopathy | HIV Encephalopathy | Improved with ART therapy |
| 5 | VGKC LGI-1 |
0.76 mmol/L |
F | 70 | No | Yes, bilateral mesotemporal lobe T2 changes | Limbic Encephalitis | Steroid-responsive |
Patient 1 was a 71 year-old woman with stage 1 colon cancer who presented with double vision and was found to have multiple areas of restricted diffusion in her brainstem on MRI. A serum paraneoplastic antibody panel was sent and returned a positive calcium channel antibody against P/Q antibody at a titer of 0.07nmol/dL (reference <=0.02). She underwent a lumbar puncture which showed a protein of 45 mg/dL, white blood cell count of 3 cells per uL, red blood cell count of 178 cells per uL and glucose of 97mg/dL. There were no oligoclonal bands in the CSF and IgG index was 0.56. A CSF paraneoplastic panel was negative. A PET of the body was negative. She did not have an EEG. The patient underwent biopsy of brain lesions which were consistent with CNS lymphoma. She was treated with 1 dose of intravenous solumedrol while awaiting the results of brain biopsy. The antibody panel result was considered a false positive.
Patient 2 was a 68 year-old woman with a 15 year history of bipolar disorder who presented with altered mental status. Serum autoimmune panel was positive for NMDA receptor antibody at a 1:10 dilution. Higher titers of antibody were not detected. The NMDA receptor antibody was also individually sent to another testing site and was negative. She underwent a lumbar puncture which showed a protein of 21 mg/dL, white blood cell count of 1 cell per uL, red blood cell count of 1 cells per uL and a glucose of 64 mg/dL. Oligoclonal bands and IgG index were not sent in the CSF. CSF autoimmune panel was negative. An MRI of the brain which was notable for non-specific white matter changes thought to be the result of small vessel disease. EEG showed bilateral slowing with no epileptiform abnormality attributable to receiving lorazepam due to concern for catatonia. She received no immunotherapy. Her psychiatric medications were titrated and she returned to her premorbid baseline thus the etiology of her condition was thought to be psychiatric.
Patient 3 was a 45 year-old man who presented with 7 months of confusion and progressive left leg weakness throughout the left leg worst at the hip. Brain MRI showed extensive symmetric white matter disease involving the corpus callosum and bilateral crus cerebri. Serum autoimmune panel showed a positive anti-GAD65 antibody with a titer of 0.06 nmol/L (reference <=0.02 nmol/L). A note is made by the lab that GAD65 antibody values less than 2.00 nM have a lower positive predictive value for neurological autoimmunity than values of 20.0 nM and higher and the reported level of 0.06 nmol/L has a lower specificity for autoimmune encephalitis. Lumbar puncture was performed and showed protein of 31 mg/dL, white blood cell count of 1 cells per uL, red blood cell count of 1 cell per uL and a glucose of 46 mg/dL. The IgG index was 0.5 and there were 3 oligoclonal bands that were also present in the serum. A CSF HIV viral load was 48,764 copies/mL. A CSF panel was not sent. EEG showed non-specific slowing. He was ultimately found to be HIV positive and started on anti-retroviral therapy. No immunotherapy was given. His presentation was consistent with a new diagnosis of HIV and HIV encephalopathy and myelopathy.
Patient 4 was a 79 year-old woman who presented with rapidly worsening memory over a 1 year period. Brain MRI showed non-specific white matter changes. Serum autoimmune panel showed a positive anti-GAD65 antibody with at a titer of 0.04 nmol/L (reference <=0.02 nmol/L). CSF showed protein of 58 mg/dL and no white blood cells, 1 red blood cell per uL and a glucose of 78 mg/dL. CSF autoimmune panel was negative. EEG showed non-focal slowing. No immunotherapy was given. She was felt to have a non-AD dementia by a dementia specialist at follow-up.
Patient 5 was a true positive case. She was a 72 year-old woman who presented with a few months of rapidly progressive memory difficulties and confusion. Her sodium was 129 on her general laboratory workup on admission. A brain MRI showed bilateral mesial temporal lobe hyperintensities. A serum autoimmune panel was positive for leucine-rich, glioma inactivated 1 protein IgG (LGI1) antibodies at a titer of 0.76 nmol/L (reference <=0.02 nmol/L). Her CSF showed a protein of 49 mg/dL, glucose of 74 mg/dL, red blood cell count of 40mg/dL and 1 white blood cell per uL. There were no oligoclonal bands in the CSF and the IgG index was 0.48. A CSF autoimmune panel was negative. A 24-hour EEG was normal but given clinical concern for seizures she received lamotrigine. A PET body was negative. She was treated with intravenous methylprednisolone for 5 days with improvement in her mental status. She was then started on rituximab and she returned to her pre-hospitalization clinical baseline.
Thus, overall 1 patient had a clinical autoimmune syndrome attributable to detected antibodies and 1 patient had antibodies and were found to have a malignancy at 1 year of follow-up.
Of those with negative serum and CSF panels, 14 were treated empirically with immunotherapy (steroids, intravenous immunoglobulin, or rituximab), 1 was treated with chemotherapy, and the rest (31) were treated with supportive care. Of those treated acutely with immunotherapy overall, 14/15 (93%) were treated before the panel tests resulted. Four patients who had negative panels but were empirically treated and met consensus criteria for an autoimmune-mediated neurologic process. The final diagnoses for all patients that panels were sent on consisted of: seizures from non-immune causes (13), infectious causes (10), toxic/metabolic causes (2), limbic encephalitis (1), sarcoidosis (2), immune-mediated encephalitis (1), immune-mediated myelitis (2), immune-mediated peripheral neuropathy or radiculopathy (3), neurodegenerative (5), neoplastic (4), and other (7). Of the entire cohort of 1,604 patients, there was one case which on long-term follow-up was felt to have an antibody-mediated neurological syndrome though antibody testing was not sent.
Conclusion
In this retrospective descriptive study we found low rates of true positive results amongst patients tested for an antibody-mediated neurologic process while admitted to the inpatient neurology service. Additionally, we found that due to acuity of presentation, the results of antibody testing did not appear to influence initial treatment decisions.
The presentations for antibody mediated neurologic disease are highly variable and can be challenging to diagnose. The advent of paraneoplastic and autoimmune antibody panels has simplified broad testing for these diagnoses. However, these advances in testing have created a need for further education regarding understanding and interpreting results within a clinical context. We investigated the number of autoimmune panels sent on patients seen on our inpatient service over the course of 1 year. We found that amongst 1,604 patients admitted over the course of 1 year, fifty patients had antibody testing sent and only 1 patient had a true positive result. During the inpatient stay most patients were treated prior to the return of their antibody test result, suggesting that antibody testing did not impact clinical decision-making. Out of 50 patients tested, there were 4 patients (8%) that had negative antibody panels but were ultimately felt to have an antibody-mediated disease that met diagnostic criteria and was responsive to steroids. There were also 4 patients (8%) that had a positive antibody result but were not felt to have a true antibody-mediated disease.
The average age and gender distribution of patients with antibody testing sent is similar to prior studies of cohorts of patients undergoing paraneoplastic testing. 5,11 The percent of true positives was higher than in other reports with our true positive rate of 2% higher than another report of 2/500 or 0.04% 5 but lower than 1 group which reported 17/401 true positives (4%). 12 We wonder if this may be related to the backgrounds of the physicians who sent testing. In the case of the group with more true positives, they were neurologists at a center with expertise in autoimmune neurology. In the case of our study, all neurologists ordering tests had experience with immune-mediated disease, though were not necessarily experts in the diagnosis and treatment of autoimmune neurologic disorders. Other works have suggested that antibody testing ordered by non-neurologists have a low yield of true positive tests (0/71 sent). 5 Taken together, this suggests the experience of the provider in diagnosing this type of disease may influence their appropriate ordering of testing. However, it is also possible that given small numbers the rates of positive results are difficult to interpret. Future work by our group would be to investigate the yield of testing sent by non-neurologists at our center.
We have presented details of the 5 cases of positive antibody results by titer in our cohort. We feel these cases are emblematic of the challenges of interpreting the results of positive antibody testing according to cut-off values, and the need for clinical evaluation and expertise.
In Case 1, while the neurologic presentation of multifocal areas of restricted diffusion does not fit with typical P/Q antibody neurologic disease, the positive antibody test result is still relevant as around 20% of patients with positive antibodies have malignancy of differing types. 13 The results from Mayo do include the possibility of associated malignancy within the interpretation of results and the unlikely nature that this low titer represents a true neurologic presentation of P/Q antibody disease. This patient was ultimately diagnosed with lymphoma. The positive antibody result was felt to be a false positive in that the patient’s symptoms were attributable to her lymphoma. Positive P/Q antibodies have been reported in 21% of patients including patients with systemic lymphoma. 13 Some of these patients do not have a neurologic manifestation. To our knowledge there are not other reports of positive P/Q antibodies in patients with CNS lymphoma. This emphasizes how the panel results can be helpful for patients’ diagnosis and care. However, without additional insight from the Mayo, there is the potential for misinterpretation.
Cases 2-4 were all felt to have false positive antibody panels. Case 2 of a patient with psychiatric disease who had a low titer positive NMDA result, demonstrates the how low positive titers can lead to diagnostic uncertainty. Further, in this case 1 serum sample from Mayo resulted as positive but another facility resulted with negative at lower titers. Her age, comorbidities, and lack of other markers of inflammation were indicators that this was likely not immune-mediated and her improvement without immunosuppression and with psychiatric medication titration also allowed to exclude this possibility. Cases 3 and 4 both have low positive antibody titers and a note is made in their results that this low degree of positivity had a low specificity for autoimmune encephalopathy and that GAD65 antibody values less than 2.0 nM have a lower positive predictive value for neurologic autoimmunity. The note of how to interpret the results within context with the lack of clinical correlation with known neurologic manifestations GAD65 and patient 3 and 4’s presentation allowed us to interpret these results.
Case 5 was thought to be an autoimmune/paraneoplastic process prior to the results of antibody panel testing given expected subacute onset of symptoms, exclusion of other causes including infection, and inflammation involving temporal parenchyma seen on MRI. The specific auto-antibody identified corresponded with her presentation of altered cognition, memory difficulty, and hyponatremia. 14 The positive result of the test did not change the empiric use of steroids in the hospital as the result had not returned, however may have influenced the long term decision to treat with rituximab given the risk of reoccurrence reported in clinic notes as up to 30%. 15
We additionally noted that after 1 year of follow-up patients followed in our outpatient clinics with positive panels did not receive routine malignancy screening at follow-up, or in some cases even at initial assessment. While we think there is benefit to having a dedicated group of clinicians (i.e., neurohospitalists) involved in the care of these patients, there is a risk of fragmented care and discontinuity during the transition from inpatient care to ambulatory follow up. In some instances, positive results could have been missed if they returned after the patient had been discharged. Non-synchronous release of results on the panels may reduce the risk of missed results. In other cases, patients did not follow-up in our clinics as they were referred from remote hospitals and sought follow up care closer to home.
The majority of our patients who were felt clinically to have an autoimmune process were treated empirically before the results of the laboratory studies became available. This suggests that the results of antibody testing was not the main driver of a decision to initiate treatment. Rather, if there is clinical concern for an antibody-mediated disease, prompt empiric treatment may be helpful while awaiting further diagnostic test results. We noted that 4 of our patients ultimately met criteria for possible immune-mediated neurologic process and benefitted clinically from immunotherapy despite a lack of antibody positivity. We noted a higher frequency of encephalopathy amongst patients who had antibody testing sent by our group, yet the 4 cases in which we felt there was an immune-mediated process based on clinical features were all peripheral nervous system or spinal-cord syndromes. Myelitis is a common autoimmune neurologic syndrome, and often seronegative. This suggests the importance of ongoing surveillance for immune-mediated disease with yet-to-be identified antibodies. We anticipate that more antibody biomarkers will emerge but in the interim patients are best treated empirically based on clinical features.
Both a paraneoplastic and autoimmune panel was sent in 4 patients, compared to just one of the 2 panels sent. Given the overlap between these panels, this may suggest problems in understanding which antibodies are included on which panel, or difficulty ordering individual tests not included in the panel. This may also add to the additional cost of testing amongst these patients.
There are many limitations to this study. First, given the small numbers, this is a descriptive analysis. Given that CSF and serum panel testing is known to increase the yield of detecting pathologic antibodies when sent together, we support the practice of sending both in patients suspected of having an autoimmune etiology of their neurologic symptoms. 16 We also recognize our small sample size as a limitation. Given the small number of patients in the study this may not be generalizable. We also acknowledge that due to the retrospective nature of this study we have may have missed some patients with antibody panels sent. We also have only followed these patients over 2 years; further longitudinal follow-up may provide further insights to their care.
In summary, the use of antibody panels has important implications for ongoing neurologic care, though may be less useful in guiding initial treatment decisions during the early diagnostic phase on an inpatient service, when decisions must be made rapidly. The rate of true positive panel results may be influenced by the physicians ordering the tests. Our data highlight the complexity of making a diagnosis of an antibody-mediated neurologic condition and the need for ongoing education surrounding these conditions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kristin Galetta  https://orcid.org/0000-0002-3783-9788
https://orcid.org/0000-0002-3783-9788
Joshua P. Klein  https://orcid.org/0000-0001-7685-2523
https://orcid.org/0000-0001-7685-2523
Shamik Bhattacharyya  https://orcid.org/0000-0001-8047-3622
https://orcid.org/0000-0001-8047-3622
References
- 1. Lancaster E, Dalmau J. Neuronal autoantigens—pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8(7):380–390. doi:10.1038/nrneurol.2012.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Byrne S, Walsh C, Hacohen Y, et al. Earlier treatment of NMDAR antibody encephalitis in children results in a better outcome. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e130. doi:10.1212/NXI.0000000000000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giometto B, Grisold W, Vitaliani R, et al. Paraneoplastic neurologic syndrome in the PNS Euronetwork Database: a European study from 20 centers. Arch Neurol. 2010;67(3):330–335. doi:10.1001/archneurol.2009.341 [DOI] [PubMed] [Google Scholar]
- 4. Grativvol RS, Cavalcante WCP, Castro LHM, Nitrini R, Simabukuro MM. Updates in the diagnosis and treatment of paraneoplastic neurologic syndromes. Curr Oncol Rep. 2018;20(11):92. doi:10.1007/s11912-018-0721-y [DOI] [PubMed] [Google Scholar]
- 5. Ebright MJ, Li SH, Reynolds E, et al. Unintended consequences of Mayo paraneoplastic evaluations. Neurology. 2018;91(22):e2057–e2066. doi:10.1212/WNL.0000000000006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albadareen R, Gronseth G, Goeden M, Sharrock M, Lechtenberg C, Wang Y. Paraneoplastic autoantibody panels: sensitivity and specificity, a retrospective cohort. Int J Neurosci. 2017;127(6):531–538. doi:10.1080/00207454.2016.1207644 [DOI] [PubMed] [Google Scholar]
- 7. Sæther SG, Schou M, Stoecker W, et al. Onconeural antibodies in acute psychiatric inpatient care. J Neuropsychiatry Clin Neurosci. 2017;29(1):74–76. doi:10.1176/appi.neuropsych.16050110 [DOI] [PubMed] [Google Scholar]
- 8. Fletcher A, Sharp C, Sweeney M. Integration of an evidence-based algorithm for inpatient autoimmune and paraneoplastic neurologic syndrome autoantibody panel ordering practice to improve diagnostic evaluation quality (466). Neurology. 2020;94:466. [Google Scholar]
- 9. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi:10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59(4):499–505. doi:10.1212/wnl.59.4.499 [DOI] [PubMed] [Google Scholar]
- 11. Kim JT, Harris NS. Utilization review of paraneoplastic neurological syndrome antibody screening panels: experience at a tertiary academic health center. J Appl Lab Med. 2019;4(1):19–29. doi:10.1373/jalm.2018.028480 [DOI] [PubMed] [Google Scholar]
- 12. Abboud H, Rossman I, Mealy MA, et al. Neuronal autoantibodies: differentiating clinically relevant and clinically irrelevant results. J Neurol. 2017;264(11):2284–2292. doi:10.1007/s00415-017-8627-4 [DOI] [PubMed] [Google Scholar]
- 13. Zalewski NL, Lennon VA, Lachance DH, Klein CJ, Pittock SJ, Mckeon A. P/Q- and N-type calcium-channel antibodies: oncological, neurological, and serological accompaniments. Muscle Nerve. 2016;54(2):220–227. doi:10.1002/mus.25027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graus F, Saiz A, Lai M, et al. Neuronal surface antigen antibodies in limbic encephalitis. Clin Immunol Assoc. 2008;71(12):930–936. doi:10.1212/01.wnl.0000325917.48466.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li W, Wu S, Meng Q, et al. Clinical characteristics and short-term prognosis of LGI1 antibody encephalitis: a retrospective case study. BMC Neurology. 2018;18(1):96. doi:10.1186/s12883-018-1099-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKeon A, Pittock SJ, Lennon VA. CSF complements serum for evaluating paraneoplastic antibodies and NMO-IgG. Neurology. 2011;76(12):1108–1110. doi:10.1212/WNL.0b013e318211c379 [DOI] [PMC free article] [PubMed] [Google Scholar]