Version Changes
Revised. Amendments from Version 2
In this new version, we provide the revision of some parts in abstract, introduction, methods, results, discussion, and figure 1. The revision of our new version does not change the main findings of our study.
Abstract
Background: Convalescent plasma (CCP) has been used for treating some infectious diseases; however, the efficacy of CCP in coronavirus disease 2019 (COVID-19) remains controversial. The aim of this research was to assess the efficacy of CCP as an adjunctive treatment in COVID-19 patients.
Methods: Embase, PubMed, Web of Science, Cochrane and MedRix were searched for potentially relevant articles. All included papers were assessed for the quality using modified Jadad scale and Newcastle-Ottawa scale for randomized controlled trial (RCT) and non – RCT, respectively. We used a Q test and Egger test to assess the heterogeneity and publication bias among studies, respectively. Mortality rates between patients treated with standard treatment and standard treatment with CCP were compared using a Z test.
Results: A total of 12 papers consisting of three cross-sectional studies, one prospective study, five retrospective studies, and three RCT studies were included in our analysis. Of them, a total of 1,937 patients treated with CCP and 3,405 patients without CCP were included. The risk of mortality was 1.92-fold higher in patients without CCP compared to patients treated with CCP (OR: 1.92; 95%CI: 1.33, 2.77; p=0.0005). In severe COVID-19 sub-group analysis, we found that patients without CCP had a 1.32 times higher risk of mortality than those treated with CCP (OR: 1.32; 95%CI: 1.09, 1.60; p=0.0040).
Conclusions: CCP, as adjunctive therapy, could reduce the mortality rate among COVID-19 patients.
Keywords: convalescent plasma, passive immunization, COVID-19, mortality, outcomes
Introduction
The management of coronavirus disease 2019 (COVID-19) remains challenging. While the guideline for the management of COVID-19 has been established, 1- 3 some reports still reported high mortality rate among COVID-19 patients. 4, 5 The guideline suggests that several treatments, including antiviral, hydroxychloroquine, steroid, anticoagulation, and other supportive treatments, should be used to treat patients with COVID-19. 1- 3 However, recent evidence from large scale studies failed to clarify the efficacy of those suggested treatments. 6- 8 Moreover, the findings from the World Health Organization (WHO) solidarity trials also failed to clarify the benefits of hydroxychloroquine, remdesivir, interferon, and lopinavir in the management of COVID-19. 8 Therefore, new approaches to COVID-19 management are required.
Convalescent plasma (CCP), an immunological therapy, is suggested to have promising efficacy for managing several infectious diseases. 9 CCP, a strategy of passive immunization, was first introduced by von Behring and Kitasato in 1890. Initially, it was used to treat diphtheria and other infectious diseases such as scarlet fever and pertussis. 10 Moreover, due to its good efficacy, this therapy was also used for the management of Ebola, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS). 11 In patients with MERS, SARS, and Ebola, the clinical improvement and reduced mortality rate were observed in patients receiving CCP compared to patients without CPP. 12 However, the efficacy of CCP against COVID-19 is conflicting. Furthermore, the previous meta-analyses resulted in inconclusive findings due to the lack of structured methodology. Therefore, a holistic meta-analysis is needed to provide insight into the clinical efficacy of CCP for the management of COVID-19.
Methods
Study design
A systematic review and meta-analysis covering the period July 2020 - December 2020 was conducted to assess the efficacy of CCP as an adjunctive treatment in COVID-19 patients. Studies from prominent bibliographic databases were searched, and the protocols followed the guideline from Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA). 13
Eligibility criteria
Relevant articles were assessed for inclusion and exclusion criteria before the final analysis. Our analysis included articles with the following criteria: (1) observational or randomized controlled trial studies; (2) providing sufficient data of COVID-19 diagnosis methods; and (3) well-identified methodologies represented with Newcastle-Ottawa Scale (NOS). Case reports, case series, letters to the editor, reviews, commentaries, low method quality, and those with pre-post test comparison were excluded.
Search strategy and data extraction
Relevant studies in four bibliographic databases (Embase, PubMed, Web of Science, and Cochrane) and a preprint database MedRix were searched as of 2 December 2020. The searches limited to English only using Medical Subjects Heading: (“COVID-19” OR “SARS-CoV-2”) AND (“convalescent plasma” OR “serotherapy” OR “hyperimmune globulin therapy” OR “convalescent plasma treatment”). A reference list of the relevant articles was also retrieved for additional references. If a duplicate publication was found, the article with the larger sample size was included. Information of: (1) name of the first author; (2) year of publication; (3) country of origin; (4) sample size of cases and controls, (5) CCP administration, and (6) mortality rate were collected from each article. Search strategy and data extraction were conducted by three independent investigators (MI, AAA & YP) using a pilot form. Disagreements were resolved in group discussions through a consensus approach. Before collecting the data, the investigators performed a discussion to define the study variables and the study protocols, and the understanding among the investigators was assessed using kappa test.
Assessment of the methodology quality
All included papers were assessed for the quality using modified jadad scale for randomized controlled trial (RCT) and Newcaste-Ottawa scale for non-RCT. 14 The quality of the articles was classified as low, moderate, and high quality. Articles with low quality were excluded from our analysis. The assessment was carried out by three independent investigators (MI, AAA & YP), and when there was a discrepancy among the investigators, a discussion was performed with a senior researcher (JKF).
Outcome measure
The primary outcome measure was all causes of mortality among COVID-19 patients treated with and without CCP. The predictor variable was COVID-19 patients treated with CCP. A sub-group analysis was conducted based on the severity of COVID-19 patients treated with CCP (i.e. mild and severe).
Statistical analysis
The association between CCP and the reduction of the risk of mortality among COVID-19 patients was assessed using a Z test. Before assessing the association, the potency of bias and heterogeneity was assessed. To assess the risk of bias, an Egger test was employed to calculate tau-squared, and a p-value of less than 0.05 indicates that the potency of bias was found. A Q test was used to assess the heterogeneity among the included papers. The p-value of less than 0.10 was considered that heterogeneity across the studies was found, and the correlation was therefore determined using a random-effect model; otherwise, a fixed-effect model was employed. All analyses were carried out using Review Manager (Revman Cochrane, London, UK) version 5.3, and the cumulative calculation was presented using a forest plot.
Results
Studies selection and baseline characteristics of the studies
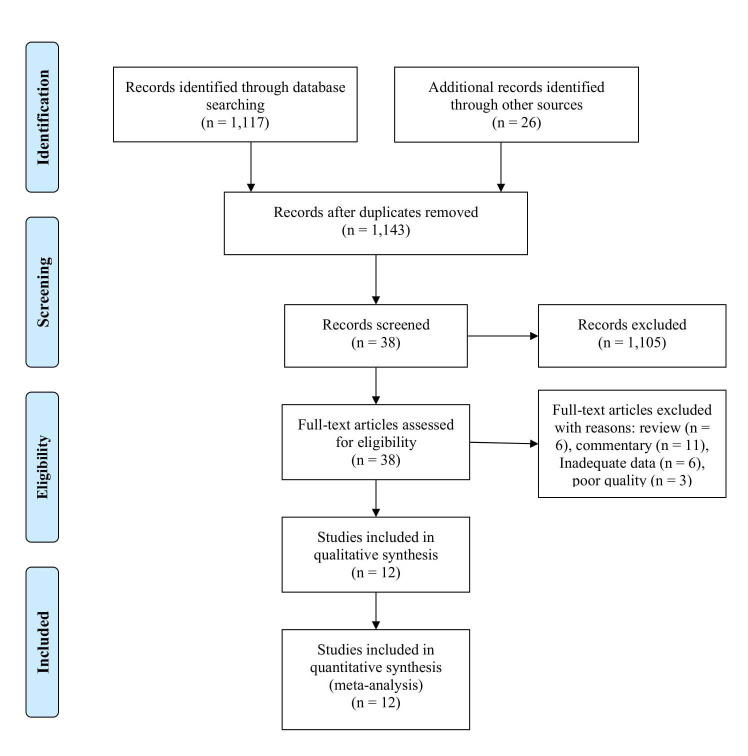
A total of 1,143 papers were identified, and 1,105 papers were excluded because they had irrelevant topics. A total of 38 papers were included for review in full-text, and 26 additional papers were excluded because of review, pre-post test model, commentary, and low-quality papers. In the final process, 12 papers were included in our analysis, consisting of three cross-sectional studies, one prospective study, five retrospective studies, and three RCT studies. 15- 26 The article selection flowchart is depicted in Figure 1, and the study characteristics are presented in Table 1.
Figure 1. A flowchart of study selection in our meta-analysis.
Table 1. Baseline characteristics of articles included in our meta-analysis.
| Name | Country | Study design | City | Sample size | CCP volume | Recipient | Quality assessment | |
|---|---|---|---|---|---|---|---|---|
| CCP | Control | |||||||
| Abolghassemi et al 2020 | Iran | Cross‐sectional | Mixed | 115 | 74 | 500 mL | Mild and severe cases | High |
| Altuntas et al 2020 | Turkey | Retrospective | Mixed | 888 | 888 | 200 - 600 mL | Severe cases | High |
| Chen et al 2020 | China | Retrospective | Hangzhou | 19 | 10 | 200-500 mL | Severe cases | Moderate |
| Gharbharan et al 2020 | Netherlands | RCT | Mixed | 43 | 43 | 300 mL | Mild and severe cases | Moderate |
| Hegerova et al 2020 | USA | Retrospective | Washington | 20 | 20 | 200 mL | Severe cases | High |
| Li et al 2020 | China | RCT | Wuhan | 52 | 51 | 100 mL | Severe cases | Moderate |
| Rasheed et al 2020 | Iraq | Cross‐sectional | Bagdad | 21 | 28 | 400 mL | Severe cases | High |
| Salazar et al 2020 (a) | US | Cross‐sectional | Mixed | 321 | 582 | NA | Mild and severe cases | High |
| Salazar et al 2020 (b) | US | Prospective | Mixed | 85 | 158 | NA | Severe cases | High |
| Xia et al 2020 | China | Retrospective | Wuhan | 138 | 1430 | 200-1200 mL | Severe cases | High |
| Zeng et al 2020 | China | Retrospective | Hangzhou | 6 | 15 | 300 mL | Severe cases | High |
Note: CCP, convalescent plasma; NOS, Newcastle-ottawa scale.
CCP efficacy against COVID-19
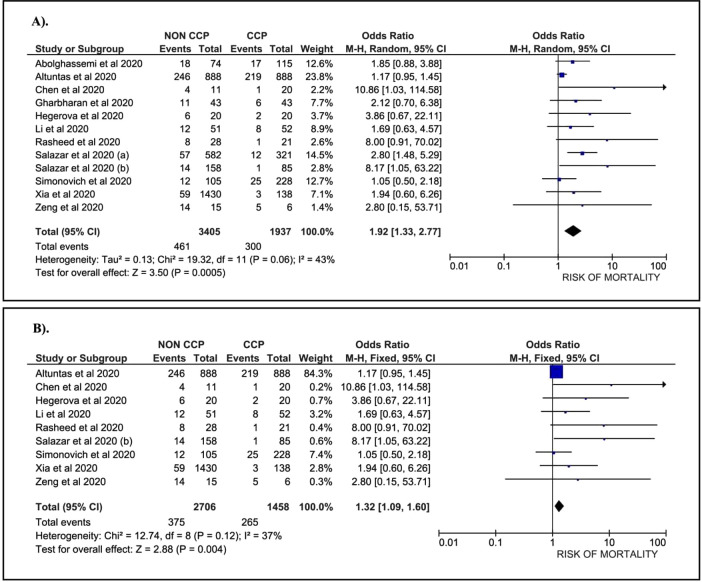
A total of 1,937 patients treated with CCP and 3,405 patients without CCP, collected from 12 papers, were included in our analysis. Data suggest that COVID-19 patients without the CCP had a 1.92-fold higher risk of mortality than patients treated with the CCP (OR: 1.92; 95%CI: 1.33, 2.77; p = 0.0005) ( Figure 2A). A sub-group analysis among severe COVID-19 patients who were treated with CCP was conducted. This sub-group consisted of nine papers with 1,458 patients treated with CCP and 2,706 patients without CCP. The data revealed a 1.32-fold higher risk of mortality in COVID-19 patients without CCP compared to patients treated with CCP (OR: 1.32; 95%CI: 1.09, 1.60; p=0.0040) ( Figure 2B).
Figure 2. Forest plot of the association between convalescent plasma and the risk of mortality.
A). All cases (OR: 1.92; 95%CI: 1.33, 2.77; p = 0.0005; p Egger: 0.3620; p Heterogeneity: 0.0600; I-squared: 43.00%). B). Severe COVID-19 (OR: 1.32; 95%CI: 1.09, 1.60; p = 0.0040; p Egger: 0.3790; p Heterogeneity: 0.1200; I-squared: 37.00%).
Heterogeneity and potency of bias across the studies
The analysis revealed evidence of heterogeneity in total case of COVID-19. Therefore, a random-effect model was applied to assess the association between CCP and the risk of mortality among COVID-19 patients. In the severe COVID-19 sub-group, we found no heterogeneity, and we used a fixed-effect model to evaluate the correlation. Our analysis using an Egger test found no publication bias in both the total and the severe COVID-19 sub-group (Funnelplot is provided in supplementary file).
Discussion
Our data suggest that CCP treatment associated with a reduction of mortality both in all cases and severe COVID-19 patients. Our current findings are consistent with the results of previous meta-analyses. 27- 32 The theory underlying the mechanism of CCP in COVID-19 patients remains open to controversy. Briefly, plasma transfer is the potential aspect that bridges the CCP and the reduced risk of mortality in COVID-19 patients. Plasma consists of various immunity components, including antibodies, anti-inflammatory cytokines, clotting and or anti-clotting factors, albumin, and protein C and S. 33, 34 It is believed that CCP in COVID-19 may modulate the immune response through antiviral effects and has immunomodulatory effects. 35 Antiviral effects of CCP may occur through neutralizing antibodies, and it was reported that IgG and IgM anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were the primary isotype antibodies identified from COVID-19 patients treated with CCP. 36 This humoral immune response may inhibit protein S of SARS-CoV-2. 37 Thereafter, they may exert the protective effects against COVID-19. The immunomodulatory effects of CCP may occur through the neutralization of cytokines and complements. 35, 38 These effects may inhibit the overactive immune system, including cytokine storm, complement activation, and hypercoagulable state regulation. 39 These mechanisms may be responsible for causing clinical improvement of COVID-19 patients. Of them, it was considered that immunoglobulin transfer is the essential factor in modulating the protective effect of CCP. 40 In SARS and influenza, it was reported that immunoglobulin transfer plays a vital role in governing clinical improvement. 9, 11 Moreover, in MERS, the CCP administration with the titers of antibodies 1:80 provided a significant immune response, and the titers of antibodies 1:40 did not provide a similar response. 41 Additionally, in Ebola, MERS, and SARS, the antibodies from the CCP may bind to the CD4 binding site on the viral envelope, and therefore may reduce the viral load and the risk of infection of the new cells. 42 It was also supported by previous studies that antibody titers from CCP donors also governed the clinical improvement of COVID-19 patients treated with CCP, 43, 44 suggesting that antibody transfer might influence the outcomes of clinical improvement.
Six systematic-reviews assessing the role of CCP in COVID-19 have been reported ( Table 2). 27- 32 However, they had some significant limitations: (a) the systematic reviews involved had a small sample size while in our current study, we had a relatively larger sample size; (b) some studies did not perform meta-analysis calculations to synthesize the data 27, 29 ; (c) some studies included several case reports and case series 28, 29 in which should be excluded in the meta-analysis 13 ; (d) previous meta-analyses assessed the role of CCP in similar infectious diseases (SARS and influenza), and the results were implemented to the case of COVID-19 30, 31 ; and (e) previous meta-analyses performed a mixed calculation where the data of the case vs. control model were combined with the data of pre-post intervention models, which might provide a high risk of bias due to the final effect that might be caused by other interventions. 29, 32 In the present meta-analysis, we only calculated the model of the case (standard treatment and CCP) vs. control (standard treatment only) and therefore might provide a better correlation.
Table 2. Previous systematic review and meta-analyses and some potential limitations.
| Author & year | Number of studies | Sample size | Potential limitations |
|---|---|---|---|
| Bakhtawar et al 2020 | 10 | 156 |
|
| Devasenapathy et al 2020 | 6 | 431 |
|
| Rabelo-da-Ponte et al 2020 | 5 | 75 |
|
| Rajendran et al 2020 | 5 | NA |
|
| Sarkar et al 2020 | 7 | 5444 |
|
| Sun et al 2020 | 15 | 1879 |
|
Note: NA, Not available; CCP, convalescent plasma.
In the present study, we emphasized that CCP provided good efficacy to reduce the risk of mortality among COVID-19 patients. Our findings might contribute to better management of COVID-19 patients, particularly to prevent the risk of mortality. It is expected that a medical council should elaborate on the standard procedures of CCP, including the dosage, donor criteria, side effects management, and post-intervention management. Since early administration of CCP provided better clinical outcomes than those with later intervention, 45 the appropriate time of CCP administration should be determined, and further studies are warranted.
Several important limitations of this study should be discussed. Some confounding factors that might govern the final outcomes were not controlled, including the immunological status, the dosage of CCP, time of intervention, donor criteria, the titers of antibodies, comorbidities, and transmission area. The majority of the included papers were retrospective studies, and therefore a further meta-analysis of randomized-controlled trials with a bigger sample size might provide a better conclusion.
Conclusion
Administration of the CCP is associated with a lower risk of mortality among COVID-19 patients compared to those without CCP, and this highlights its potency to be used for the treatment of COVID-19. However, studies are warranted to formulate the dosage, time of intervention, donor criteria, and the titers of antibodies to optimize the effects.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Reporting guidelines
Figshare: PRISMA checklist for ‘Association between convalescent plasma and the risk of mortality among patients with COVID-19: A meta-analysis’, https://doi.org/10.6084/m9.figshare.13490541.v1. 46
Extended data
The supplementary file regarding the funnel plot of our study is provided in Figshare ( https://doi.org/10.6084/m9.figshare.14046254.v1). 47
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgements
We thank to Lembaga Pengelola Dana Pendidikan (LPDP) Republik Indonesia for supporting this project.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 3; peer review: 2 approved]
References
- 1. Lamontagne F, Agoritsas T, Macdonald H, et al. : A living WHO guideline on drugs for covid-19. BMJ 2020;370:m3379. 10.1136/bmj.m3379 [DOI] [PubMed] [Google Scholar]
- 2. Falavigna M, Colpani V, Stein C, et al. : Guidelines for the pharmacological treatment of COVID-19. The task-force/consensus guideline of the Brazilian Association of Intensive Care Medicine, the Brazilian Society of Infectious Diseases and the Brazilian Society of Pulmonology and Tisiology. Rev Bras Ter Intensiva 2020;32:166–96. 10.5935/0103-507x.20200039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jin YH, Cai L, Cheng ZS, et al. : A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020;7:4. 10.1186/s40779-020-0233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baud D, Qi X, Nielsen-Saines K, et al. : Real estimates of mortality following COVID-19 infection. Lancet Infect Dis 2020;20:773. 10.1016/S1473-3099(20)30195-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karimullah MDH, Niazta NA, Ardining H: Venous Thromboembolism Prevention in COVID-19: A Review of Latest Evidences. Heart Science J 2020;1:10–14. 10.21776/ub.hsj.2020.001.03.3 [DOI] [Google Scholar]
- 6. Singh AK, Singh A, Singh R, et al. : Hydroxychloroquine in patients with COVID-19: A Systematic Review and meta-analysis. Diabetes Metab Syndr 2020;14:589–96. 10.1016/j.dsx.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Zhang D, Du G, et al. : Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569–78. 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Consortium WHOST. Pan H, Peto R, et al. : Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med 2020. 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeh KM, Chiueh TS, Siu LK, et al. : Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother 2005;56:919–22. 10.1093/jac/dki346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaufmann SH: Remembering Emil von Behring: from Tetanus Treatment to Antibody Cooperation with Phagocytes. mBio 2017;8. 10.1128/mBio.00117-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liya G, Yuguang W, Jian L, et al. : Studies on viral pneumonia related to novel coronavirus SARS-CoV-2, SARS-CoV, and MERS-CoV: a literature review. APMIS 2020;128:423–32. 10.1111/apm.13047 [DOI] [PubMed] [Google Scholar]
- 12. Marano G, Vaglio S, Pupella S, et al. : Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus 2016;14:152–7. 10.2450/2015.0131-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, et al. : The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stang A: Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 15. Abolghasemi H, Eshghi P, Cheraghali AM, et al. : Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus Apher Sci 2020;59:102875. 10.1016/j.transci.2020.102875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altuntas F, Ata N, Yigenoglu TN, et al. : Convalescent plasma therapy in patients with COVID-19. Transfus Apher Sci 2020;102955. 10.1016/j.transci.2020.102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen B, Xia R: Early experience with convalescent plasma as immunotherapy for COVID-19 in China: Knowns and unknowns. Vox Sang 2020;115:507–14. 10.1111/vox.12968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gharbharan A, Jordans CC, GeurtsvanKessel C, et al. : Convalescent Plasma for COVID-19. A randomized clinical trial. MEDRxiv 2020. 10.1101/2020.07.01.20139857v1 [DOI] [Google Scholar]
- 19. Hegerova L, Gooley TA, Sweerus KA, et al. : Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood 2020;136:759–62. 10.1182/blood.2020006964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li L, Zhang W, Hu Y, et al. : Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA 2020;324:460–70. 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rasheed AM, Fatak DF, Hashim HA, et al. : The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med 2020;28:357–66. [PubMed] [Google Scholar]
- 22. Salazar E, Christensen PA, Graviss EA, et al. : Significantly Decreased Mortality in a Large Cohort of Coronavirus Disease 2019 (COVID-19) Patients Transfused Early with Convalescent Plasma Containing High-Titer Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein IgG. Am J Pathol 2020. 10.1016/j.ajpath.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salazar E, Christensen PA, Graviss EA, et al. : Treatment of Coronavirus Disease 2019 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality. Am J Pathol 2020;190:2290–303. 10.1016/j.ajpath.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xia X, Li K, Wu L, et al. : Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood 2020;136:755–9. 10.1182/blood.2020007079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeng QL, Yu ZJ, Gou JJ, et al. : Effect of Convalescent Plasma Therapy on Viral Shedding and Survival in Patients With Coronavirus Disease 2019. J Infect Dis 2020;222:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simonovich VA, Burgos Pratx LD, Scibona P, et al. : A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med 2020. 10.1056/NEJMoa2031304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajendran K, Krishnasamy N, Rangarajan J, et al. : Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol 2020;92:1475–83. 10.1002/jmv.25961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rabelo-da-Ponte FD, Silvello D, Scherer JN, et al. : Convalescent Plasma Therapy in Patients With Severe or Life-Threatening COVID-19: A Metadata Analysis. J Infect Dis 2020;222:1575–8. 10.1093/infdis/jiaa509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bakhtawar N, Usman M, Khan MMU: Convalescent Plasma Therapy and Its Effects On COVID-19 Patient Outcomes: A Systematic Review of Current Literature. Cureus 2020;12:e9535. 10.7759/cureus.9535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Devasenapathy N, Ye Z, Loeb M, et al. : Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systematic review and meta-analysis. CMAJ 2020;192:E745–E55. 10.1503/cmaj.200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun M, Xu Y, He H, et al. : A potentially effective treatment for COVID-19: A systematic review and meta-analysis of convalescent plasma therapy in treating severe infectious disease. Int J Infect Dis 2020;98:334–46. 10.1016/j.ijid.2020.06.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sarkar S, Soni KD, Khanna P: Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J Med Virol 2020. 10.1002/jmv.26408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rojas M, Rodriguez Y, Monsalve DM, et al. : Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev 2020;19:102554. 10.1016/j.autrev.2020.102554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mudatsir M, Fajar JK, Wulandari L, et al. : Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Res 2020;9:1107. 10.12688/f1000research.26186.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alijotas-Reig J, Esteve-Valverde E, Belizna C, et al. : Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun Rev 2020;19:102569. 10.12688/f1000research.26186.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dulipsingh L, Ibrahim D, Schaefer EJ, et al. : SARS-CoV-2 serology and virology trends in donors and recipients of convalescent plasma. Transfus Apher Sci 2020;102922. 10.1016/j.transci.2020.102922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xi Y: Convalescent plasma therapy for COVID-19: a tried-and-true old strategy? Signal Transduct Target Ther 2020;5:203. 10.1038/s41392-020-00310-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Focosi D, Anderson AO, Tang JW, et al. : Convalescent Plasma Therapy for COVID-19: State of the Art. Clin Microbiol Rev 2020;33. 10.1128/CMR.00072-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jaiswal V, Nasa P, Raouf M, et al. : Therapeutic plasma exchange followed by convalescent plasma transfusion in critical COVID-19-An exploratory study. Int J Infect Dis 2020;102:332–4. 10.1016/j.ijid.2020.10.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Langhi DMJ, Santis GC, Bordin JO: COVID-19 convalescent plasma transfusion. Hematol Transfus Cell Ther 2020;42:113–5. 10.1016/j.htct.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ko JH, Seok H, Cho SY, et al. : Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther 2018;23:617–22. 10.3851/IMP3243 [DOI] [PubMed] [Google Scholar]
- 42. Schoofs T, Klein F, Braunschweig M, et al. : HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science 2016;352:997–1001. 10.1126/science.aaf0972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu F, Liu M, Wang A, et al. : Evaluating the Association of Clinical Characteristics With Neutralizing Antibody Levels in Patients Who Have Recovered From Mild COVID-19 in Shanghai, China. JAMA Intern Med 2020;180:1356–62. 10.1001/jamainternmed.2020.4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bradfute SB, Hurwitz I, Yingling AV, et al. : Severe Acute Respiratory Syndrome Coronavirus 2 Neutralizing Antibody Titers in Convalescent Plasma and Recipients in New Mexico: An Open Treatment Study in Patients With Coronavirus Disease 2019. J Infect Dis 2020;222:1620–8. 10.1093/infdis/jiaa505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheng Y, Wong R, Soo YO, et al. : Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005;24:44–6. 10.1007/s10096-004-1271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fajar J: PRISMA CHECLIST for Association between convalescent plasma and the risk of mortality among patients with COVID-19: A meta-analysis. figshare 2020. 10.6084/m9.figshare.13490541.v1 [DOI] [PMC free article] [PubMed]
- 47. Fajar J: Supplementary file 2. The association between convalescent plasma and the risk of mortality. figshare. Dataset 2021. 10.6084/m9.figshare.14046254.v1 [DOI] [PMC free article] [PubMed]