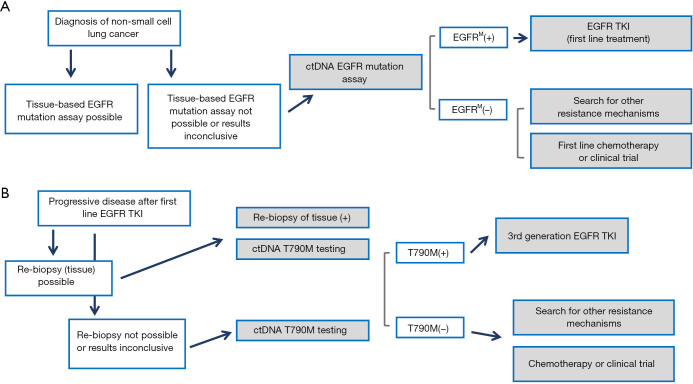
Figure 1.
Current paradigm for the use of plasma genotyping in clinical practice. (A) In patients diagnosed with NSCLC, when tissue samples cannot be used for mutation analysis, ctDNA genotyping by means of plasma analysis is recommended. EGFR tyrosine kinase inhibitor (TKI) treatment may be initiated based of plasma ctDNA testing. (B) When patients acquire resistance to first line EGFR TKI treatment, liquid biopsies can compensate tumor biopsies—given the fact that also tissues analysis is often not accurate in diagnosing the complex and heterogeneous pattern of acquired drug resistance. Figure adapted after Huang et al. (3).