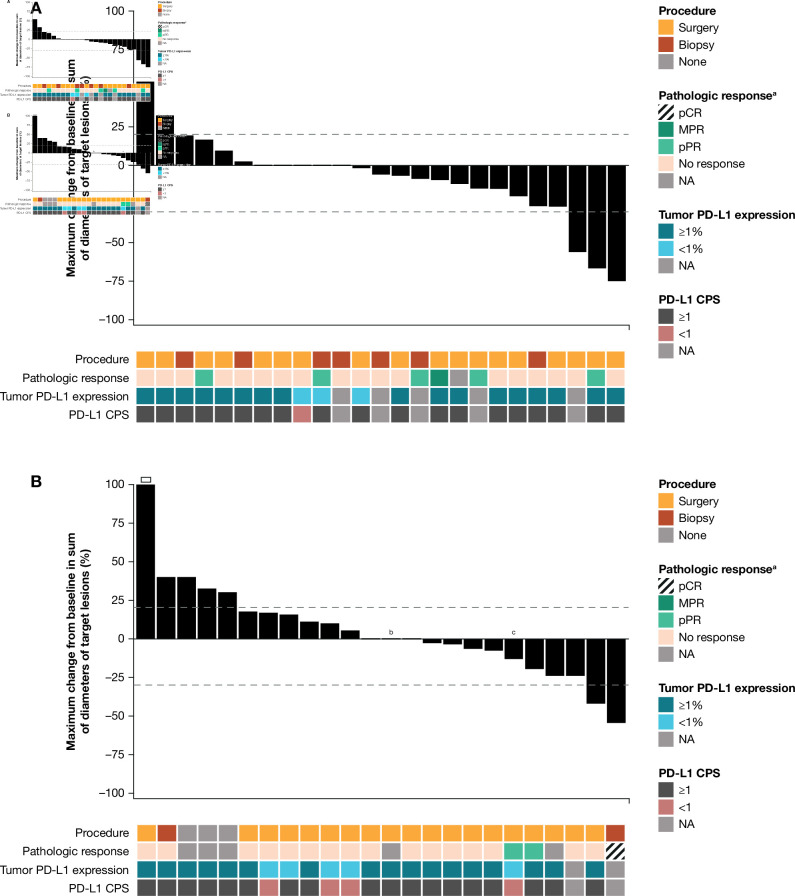
Figure 1.
Characteristics of treatment response. Change from baseline in the sum of target lesion diameters according to adapted RECIST v1.1 in evaluable patients in the (A) HPV-positive (n=25) and (B) HPV-negative (n=24) cohorts. Dashed horizontal lines indicate 30% target lesion reduction (consistent with a partial response in the absence of new lesions) and 20% increase (consistent with progressive disease). An open square indicates truncation of percent change at +100%. Note that radiographic responses were measured using adapted RECIST v1.1 comprising a single on-treatment imaging scan before surgery, with no confirmatory scan performed. Detailed per-patient data are provided in online supplemental tables S4 and S5. CPS, combined positive score; HPV, human papillomavirus; MPR, major pathologic response; NA, not available; pCR, pathological complete response; pPR, pathologic partial response; PD-L1, programmed cell death ligand 1; RECIST, Response Evaluation Criteria in Solid Tumors. aBased on central pathology review. bPatient received only one neoadjuvant dose of nivolumab. cFollowing database lock, patient was found to have received planned post-nivolumab biopsy instead of complete surgical resection.