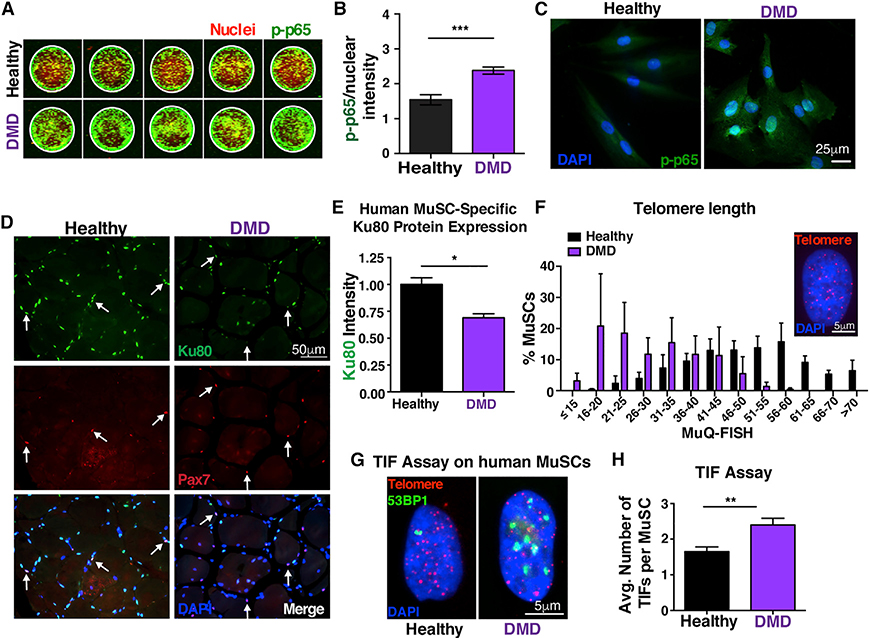
Figure 6. Persistent NF-κB activation in human DMD MuSCs leads to Ku80 dysregulation and predisposes these cells to premature telomere shortening and increased number of TIFs.
(A) In-cell western representative image of phospho-p65 staining of human MuSCs. MuSCs from three healthy and three DMD patients 10–15 years old were analyzed with five technical replicates each. Green, phospho-p65 staining; red, Draq5 (nuclear) staining.
(B) Quantification shows nuclear accumulation of p-p65 in hMuSCs. The total signal intensity in the IR 800 channel (p-p65) was normalized to the total intensity of the Draq5 (nuclear channel) per sample. n = 3 samples/group. n = 6 wells per sample.
(C) Higher NF-κB activity in NCAM+ human MuSCs from young DMD patients, as shown by the p-p65 nuclear accumulation compared to age-matched control MuSCs.
(D) n = 3 samples/group. Representative Ku80 staining (green), Pax7 (red), and nuclei (DAPI, blue) in human healthy and DMD muscle sections (21–26 years old).
(E) Quantification shows reduced levels of Ku80 in dystrophic MuSCs compared to controls. n = 3 samples/group. n > 30 cells per sample.
(F) Reduced telomere length in human MuSCs, assessed by MuQ-FISH of telomere staining (insert, representative image). n = 3 samples/group. n > 80 cells per sample.
(G) Representative images of TIF assay in human MuSCs.
(H) Quantification analysis reveals increased number of TIFs in human MuSCs.
n = 3 samples/group. n > 60 cells per sample. Graphed data are displayed as mean ± SEM. Statistical analyses in (B), (E), and (H) were performed using unpaired Student’s t test with Welch’s correction. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.