Abstract
Background:
The objective of the study is to estimate the protective role of statins in patients with acute coronary syndrome (ACS) through modulation of annexin A1 (AnxA1) serum levels.
Methods:
A total number of 63 patients with ACS were recruited compared with 25 healthy control subjects. The enrolments were divided into – Group (A): Patients with ACS on atorvastatin (n = 20), Group (B): Patients with ACS on rosuvastatin (n = 20), Group (C): Patients with ACS but not on statin therapy (n = 23), and Group (D): Healthy controls (n = 25). Body mass index and both systolic blood pressure and diastolic blood pressures were measured. Lipid profile, atherogenic index, cardiac risk ratio, cardiovascular risk index, and human AnxA1 level were estimated.
Results:
AnxA1 serum level was higher in patients with ACS (3.35 ± 0.84) compared with healthy controls (1.71 ± 0.91) and nonstatin using patients (1.47 ± 0.76) (P = 0.005).
Conclusion:
AnxA1 serum level is reduced in patients with ACS compared with healthy controls. Patients with ACS on statins therapy showed a higher level of AnxA1 compared with patients with ACS but not on statin therapy.
Key Words: Acute coronary syndrome, annexin A1, atorvastatin, rosuvastatin
INTRODUCTION
The term “acute coronary syndrome” (ACS) encompasses a range of thrombotic coronary artery diseases (CAD), including unstable angina and both ST-segment elevation and non–ST-segment elevation myocardial infarction. Diagnosis requires an electrocardiogram and a careful review for signs and symptoms of cardiac ischemia. In ACS, common electrocardiographic abnormalities include T-wave tenting or inversion, ST-segment elevation or depression (including J-point elevation in multiple leads), and pathologic Q waves.[1]
Hydroxymethylglutaryl-coenzyme A reductase inhibitors, also known as “statins,” are used adjunctively to diet and exercise to treat hypercholesterolemia by lowering total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) concentrations while increasing high-density lipoprotein cholesterol (HDL-C) concentrations.[2] The statins are indicated for the treatment and/or prevention of atherosclerotic cardiovascular disease (e.g., myocardial infarction or stroke).[3]
Statins have an important role in reducing both morbidity and mortality in patients with ACS, apart from cholesterol-lowering effect. Statins are known to have multiple effects, which are independent of cholesterol-lowering effect known as pleiotropic effects, which are antioxidant properties, enhance endothelial function, and promote atherosclerotic plaque stability, reduction of platelets aggregation, and coagulation process, and increase anti-inflammatory effect.[4]
Annexin A1 (AnxA1), formerly called lipocortin-1, is a key player in the resolution of inflammation and known as a mediator of the anti-inflammatory effects of glucocorticoids. It is abundantly expressed in innate immune cells under normal conditions. In experimental models, AnxA1 or AnxA1-derived peptides exert a broad range of anti-inflammatory effects in monocytes, involving transcriptional changes, as well as rapid posttranslational effects.[5] A number of studies have also shown that glucocorticoids induce de novo synthesis as well as translocation of AnxA1 to the cell surface in the peripheral blood mononuclear cells or isolated monocytes/macrophages.[5] The expression of AnxA1 in circulating immune cells may thus serve as an indicator of anti-inflammatory actions and glucocorticoid sensitivity.[6]
Several studies have described high plasma levels of inflammatory cytokines and enhanced activation of neutrophils in patients with CAD, particularly in those with unstable conditions of the disease. The presence of anti-inflammatory mediators, on the other hand, is less well documented.[7]
A proteomic analysis of human coronary arteries demonstrated increased levels of AnxA1 in atherosclerotic tissue compared with nonatherosclerotic tissue and further validation by immunohistochemistry suggested that AnxA1 was expressed by macrophages in the intima. Interestingly, a study of human carotid plaques reported that AnxA1 expression was increased in plaques from asymptomatic patients compared with plaques from symptomatic patients, thus indicating an association between AnxA1 and plaque stabilization.[8] Previously, Yixian and Ping found that the anti-inflammatory effect of lovastatin was mediated upregulation of AnxA1.[9]
The hypothesis and rationale of the present study depend on the ameliorating effect of statins on the AnxA1 serum level. Therefore, the aim of the present study was to investigate the effect of statin therapy on AnxA1 level in patients with ACS.
METHODS
In this case–control study, patients with ACS with or without statin therapy aged between 45 and 70 years were recruited from the coronary care unit according the American diagnostic criteria[10] compared with healthy controls. This study was approved by ethical committee and editorial board in Al-Mustansiriya University/College of Medicine, from January to March 2019 (IRB 23DT in 22/5/2019), in accordance with the ethical standards set forth in the Declaration of Helsinki in 1975. Direct interview, full history, and physical examination were done. Full routine investigations were recommended for each patient regarding previous investigations, duration and type of statin therapy, dietary habits, and life style modifications. All patients and enrolled participants gave informed verbal consent for their participation in this study. According to the statin therapy, patients with ACS and healthy controls were divided as given below:
Group A: Patients with ACS on atorvastatin
Group B: Patients with ACS on rosuvastatin
Group C: Patients with ACS but not on statins therapy
Group D: Healthy controls.
Inclusion criteria
Any patients with ACS with age >45 years with or without statin (for at least 3 months) therapy were included depending on the potential effect of statins on the human AnxA1 serum level in patients with ACS.
Exclusion criteria
Any patients with severe or morbid obesity, end-stage kidney disease, liver failure, psychiatric disorders, severe anemia, connective tissue diseases, pregnancy, lactation, and malignancy were excluded from the present study.
Anthropometric measurements
Body mass index (BMI) was obtained from measuring the weight in kilograms and the height in meters; then, BMI was calculated by specific equation, BMI = weight (kg)/height (m2).[11,12] Blood pressure (mmHg) was measured by using mercury sphygmomanometer device (MDF/Germany), in supine position, for each patient, and average of two blood pressure readings was taken. Both systolic blood pressure and diastolic blood pressure were measured.[13]
Biochemical measurements
Estimation of lipid profile
TC, TG, and HDL were measured by auto-analyzer (ERBE diagnostic Manheim, Germany). LDL was estimated by Friedewald equation.[14] very LDL (VLDL) = TG/5, atherogenic index (AI) = log (TG/HDL), cardiac risk ratio (CRR) = TC/HDL, cardiovascular risk index (CVRI) = TG/HDL.[15] Determination of human AnxA1 level was done by using ELISA kit method (Human ANXA1, MyBioSource/USA) which was expressed as ng/ml.
All biochemical measurements were done in the Laboratory Unite, Department of Clinical Pharmacology, College of Medicine, Al-Mustansiryia University, Baghdad, Iraq.
Statistical analysis
Analysis of data was carried out using the available statistical package of SPSS-24 (IBM SPSS, Stastics for Window, Version 24.00; 2019, Armonk, NY, USA: IBM Corp). Data were presented in simple measures of percentage, numbers, mean, and standard deviation. The sample size was determined as two-third of cases compared to one-third of controls. Unpaired t-test was used to test the difference between two independent means , however ANOVA test was used to find the differences among more than two independent means. Statistical significance was considered regarding P < 0.05.
RESULTS
In the present study, at first, a total number of 78 patients were recruited, 15 of them were excluded (5 with renal impairments, 4 with chronic hepatitis, and 6 with endocrine disorders); then, the 63 patients with ACS were divided as follows – 40 of them were on statin therapy (statin users) and 23 were not on statin therapy (nonstatin users) compared with 25 healthy controls. All recruited patients and healthy controls completed the study without any withdrawal as revealed in the consort flow of the present study [Figure 1].
Figure 1.
Consort flow of the present study
Demographic characteristics of the present study
The allocation of patients and control groups seems to be equally matched in respect to age, gender weight, height, and BMI; therefore, there was no significant difference in BMI among patients with or without statin therapy and controls (P = 0.52). The study revealed that 28 out of 63 patients were smokers, while in the control group, 60% were smokers and 40% were nonsmokers. The patients gave a history of other associated comorbidities that seem to increase the risk of ACS, including 31 (49.20%) patients with hypertension, 1 (1.58%) patient with familial dyslipidemia, 28 (44.44%) patients with ischemic heart disease, and 7 (11.11%) patients with cerebrovascular accident. Patients were subclassified according to their life style; 38% of patients had a sedentary lifestyle, 26.9% were of moderate activities, and 34.9% were physically active, while in the control group, 80% were patients with good physical activity and 20% were patients with moderate activities [Table 1].
Table 1.
Demographic characteristics of acute coronary syndrome patients and controls
| Variables | Controls (n=25), n (%) | Patients (n=63), n (%) | P |
|---|---|---|---|
| Age (years) | 62.64±13.6 | 62.8±9.85 | 0.52 |
| BMI (kg/m2) | 28.30±5.52 | 27.66±5.39 | 0.44 |
| Gender | |||
| Male | 18 (72) | 46 (73) | 0.32 |
| Female | 7 (28) | 17 (27) | 0.52 |
| Smoker | |||
| Yes | 15 (60) | 28 (44.4) | 0.03 |
| No | 10 (40) | 35 (55.5) | 0.04 |
| ACS subgroups | |||
| STEMI | - | 30 (47.6) | |
| NSTEMI | 22 (34.9) | ||
| UA | 11 (17.4) | ||
| PMH | |||
| Hypertension | - | 31 (49.2) | |
| Dyslipidemia | 1 (1.5) | ||
| IHD | 28 (44.4) | ||
| CVA | 7 (11.1) | ||
| Life style | |||
| Active | 20 (80) | 22 (34.9) | |
| Moderate | 5 (20) | 17 (26.9) | |
| Sedentary | - | 24 (38) |
Data are expressed as n, mean±SD, percentage. BMI: Body mass index, ACS: Acute coronary syndrome, UA: Unstable angina, STEMI: ST-elevation myocardial infarction, NSTEMI: Non-STEMI, PMH: Past medical history, CVA: Cerebrovascular accident, IHD: Ischemic heart disease, SD: Standard deviation
Assessment of metabolic profile in patients with acute coronary syndrome
Regarding the metabolic profile, TC, TG, VLDL, LDL, AI, CRR, and CVRI were higher in patients with ACS but not on statin therapy (P <0.001). Regarding HDL level, it was higher in statin users compared to nonstatin patients [Table 2].
Table 2.
Metabolic profile in patients with acute coronary syndrome regarding statins therapy
| Variables | Controls (n=25) | Statins (n=40) | Nonstatins (n=23) | P |
|---|---|---|---|---|
| TC (mg/dl) | 160.4±30.7 | 141.87±40.67 | 205.78±40.6# | 0.001 |
| HDL-C (mg/dl) | 29.5±5.4 | 33.4±9.09* | 27.4±3.96# | 0.005 |
| TG (mg/dl) | 91.8±44.66 | 133.57±37.93* | 160.65±39.9# | 0.001 |
| VLDL (mg/dl) | 18.37±8.93 | 26.71±7.58* | 32.13±7.98# | 0.001 |
| LDL-C (mg/dl) | 112.46±32.69 | 81.76±42.42* | 146.17±40.69# | 0.001 |
| AI | 0.45±0.2 | 0.59±0.17* | 0.76±0.12# | 0.001 |
| CRR | 5.65±1.71 | 4.57±1.87* | 7.66±2.08# | 0.001 |
| CVRI | 3.22±1.81 | 4.24±1.45* | 5.98±1.72# | 0.001 |
| SBP (mmHg) | 117.2±8.3 | 128.7±17.94* | 129.0±16.51 | 0.008 |
| DBP (mmHg) | 76.6±5.72 | 77.12±8.88 | 79.30±13.3 | 0.57 |
*P<0.05 compared between control with statin groups, #P<0.05 compared between statin with nonstatin groups, P<0.05 compared between controls with nonstatin groups. Data are presented as mean±SD, ANOVA test and LSDpost hoctest. TC: Total cholesterol, TG: Triglyceride, HDL-C: High-density lipoprotein cholesterol, LDL-C: Low-density lipoprotein cholesterol, VLDL: Very LDL, AI: Atherogenic index, CRR: Cardiac risk ratio, CVRI: Cardiovascular risk index, SBP: Systolic blood pressure, DBP: Diastolic blood pressures, SD: Standard deviation, LSD: Least significant difference
Effect of statins therapy on annexin A1 serum levels in patients with acute coronary syndrome
A higher AnxA1 level (3.35 ± 0.84) was obtained from patients on statin therapy in comparison to the controls (1.71 ± 0.91) and patients using nonstatin (1.47 ± 0.76) (P = 0.005) [Figure 2]. AnxA1 serum level was significantly higher in rosuvastatin (3.69 ± 0.92) compared with atorvastatin (3.00 ± 0.60) (P = 0.008) [Figure 3].
Figure 2.
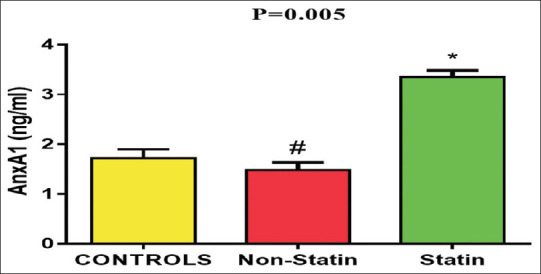
Mean of annexin A1 level in acute coronary syndrome patients. *P≤ 0.01 compared to the nonstatin and control groups. #P< 0.05 compared to the control
Figure 3.
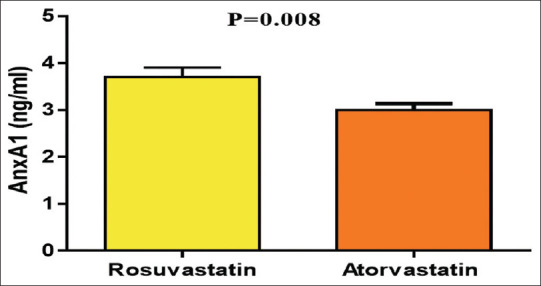
Annexin A1 serum level patients with acute coronary syndrome on atorvastatin versus rosuvastatin
DISCUSSION
The finding core of the present study was significant elevation in the AnxA1 serum level in patients on statin therapy as compared with nonstatin users' patients, as revealed by Yoshiyuki's study.[16] inflammation is linked with the development of atherosclerosis. AnxA1 inhibits pro-inflammatory response through suppression of FPRL-1/ALXR signaling pathway.[17]
AnxA1 provides a protective role in patients with ACS by inhibiting integrin activation and myeloid cells accumulation in the arterial wall, promotes plaque stability and reduces necrosis, induces neutrophils apoptosis, and decreases endothelial cell adhesion molecules, thus reducing atherogenesis.[18]
The impact of statins on AnxA1 level was reported in a previous study that showed an elevation in the expression of AnxA1 in patients with statin therapy by calcium-mediated effect.[19] A previous study demonstrated the effect of high-dose atorvastatin in modifying the protein profile of monocytes in 33 patients with ACS and revealed an increased expression of AnxA1 in those patients compared with 22 controls.[20] However, our current study highlights a significant impact of rosuvastatin on increasing AnxA1 level in patients with ACS.
The effects of rosuvastatin on enhancing AnxA1 level may be due to several indirect causes including the hydrophilic nature of the drug and its effect in improving HDL level to a greater extent than atorvastatin since HDL and Apo A lipoprotein have a significant role in increasing AnxA1 level through different signaling pathway. As well, rosuvastatin increases endothelial AnxA1 expression through augmentation of cAMP level, which involved in the synthesis and release of AnxA1.[21,22]
Limitations of the present study were small sample size that depended on the availability of patients with ACS on statins therapy; potential source of bias was not estimated; doses of statin drugs were not estimated precisely, as well doses of statins and compliance were not recorded; and finally, this study was not prospective to determine the final decision regarding the effect of statins on the AnxA1 serum levels. However, in this study, there were no any missing data since all recruited patients and controls continue the study; therefore, this study is regarded as a preliminary pilot study for large-scale prospective study.
CONCLUSION
Patients with ACS on statin therapy showed a higher level of AnxA1 compared with patients with ACS but not on statins therapy, and further studies are warranted especially comparing different statins.
Research quality and ethics statement
The authors of this manuscript declare that this scientific work complies with reporting quality, formatting and reproducibility guidelines set forth by the EQUATOR Network, notably the STROBE guidelines. The authors also attest that this clinical investigation was determined to require and approved by the Institutional Ethics Committee at Al-Mustansiriya University College of Medicine (IRB 23DT in 22/5/2019).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to express deep thanks for all under-graduate and postgraduate medical students for their participation in this experimental novel study.
REFERENCES
- 1.Al-Kuraishy HM, Al-Gareeb AI. Acylation-stimulating protein is a surrogate biomarker for acute myocardial infarction: Role of statins. J Lab Physicians. 2017;9:163–9. doi: 10.4103/0974-2727.208263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Kuraishy HM, Al-Gareeb AI. Effects of rosuvastatin alone or in combination with omega-3 fatty acid on adiponectin levels and cardiometabolic profile. J Basic Clin Pharm. 2016;8:8–14. doi: 10.4103/0976-0105.195080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Kuraishy HM, Al-Gareeb AI, Awad MS, Alrifai SB. Assessment of serum prolactin levels in acute myocardial infarction: The role of pharmacotherapy. Indian J Endocrinol Metab. 2016;20:72–9. doi: 10.4103/2230-8210.172240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Kuraishy HM, Al-Gareeb AI, Al-Buhadilly AK. Rosuvastatin improves vaspin serum levels in obese patients with acute coronary syndrome. Diseases. 2018;6:9–18. doi: 10.3390/diseases6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gobbetti T, Cooray SN. Annexin A1 and resolution of inflammation: tissue repairing properties and signalling signature. J Biol Chem. 2016;397:981–93. doi: 10.1515/hsz-2016-0200. [DOI] [PubMed] [Google Scholar]
- 6.de Jong R, Leoni G, Drechsler M, Soehnlein O. The advantageous role of annexin A1 in cardiovascular disease. Cell Adh Migr. 2017;11:261–74. doi: 10.1080/19336918.2016.1259059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhankwala SS, Gavsane AN, Ghuli PM, Jadhav SA. Review on ischemic heart diseases and its medication. Pharma Tutor. 2018;6:23–31. [Google Scholar]
- 8.Viiri LE, Full LE, Navin TJ, Begum S, Didangelos A, Astola N, et al. Smooth muscle cells in human atherosclerosis: Proteomic profiling reveals differences in expression of annexin A1 and mitochondrial proteins in carotid disease. J Mol Cell Cardiol. 2013;54:65–72. doi: 10.1016/j.yjmcc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Yixian GA, Ping XI. Effect of fluvastatin on the expression of annexin A_1 in human peripheral blood mononuclear cells. J Mol Cell Cardiol. 2006;4:66–9. [Google Scholar]
- 10.Fihn Stephan D. ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Al-Samerraie AY, Hamada MT, Al-Kuraishy HM. Effects of metformin on omentin levels in a newly diagnosed type II diabetes mellitus: Randomized, placebo controlled study. MMJ. 2016;15:49–56. [Google Scholar]
- 12.Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ. Concept and connotation of oxidative stress in preeclampsia. J Lab Physicians. 2018;10:276–82. doi: 10.4103/JLP.JLP_26_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Kuraishy HM, Khaleel KJ, Mohammed MA. Significant attenuation and amelioration effects of labetalol in doxorubicin induced cardiotoxicity: An animal model study. Cardiovas Surg. 2015;3:25–9. [Google Scholar]
- 14.Palmer MK, Barter PJ, Lundman P, Nicholls SJ, Toth PP, Karlson BW. Comparing a novel equation for calculating low-density lipoprotein cholesterol with the Friedewald equation: A VOYAGER analysis. J Biol Chem. 2019;64:24–9. doi: 10.1016/j.clinbiochem.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Al-Kuraishy HM, Al-Gareeb AI, Al-Buhadilly AK. Rosuvastatin improves vaspin serum levels in obese patients with acute coronary syndrome. Diseases. 2018;6:9. doi: 10.3390/diseases6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshiyuki M. Simvastatin up-regulates annexin a10 that can inhibit the proliferation, migration, and invasion in androgen-independent human prostate cancer cells. Prostate. 2017;77:337–49. doi: 10.1002/pros.23273. [DOI] [PubMed] [Google Scholar]
- 17.Al-Kuraishy HM, Al-Gareeb AI. Effects of rosuvastatin on metabolic profile: Versatility of dose-dependent effect. J Adv Pharm Technol Res. 2019;10:33–8. doi: 10.4103/japtr.JAPTR_330_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elshazly MB, Nicholls SJ, Nissen SE, St John J, Martin SS, Jones SR, et al. Implications of total to high-density lipoprotein cholesterol ratio discordance with alternative lipid parameters for coronary atheroma progression and cardiovascular events. Am J Cardiol. 2016;118:647–55. doi: 10.1016/j.amjcard.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Al-Naimi MS, Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI. Berberine attenuates olanzapine induced-metabolic syndrome. J Pak Med Assoc. 2019;69(Suppl 3):S88–92. [PubMed] [Google Scholar]
- 20.Al-Bachaji IN, Al-Buhadiliy AK, Al-Kuraishy HM, Al-Gareeb AI. Proton pump inhibitors regulate metabolic profile and glycaemic indices in patients with type 2 diabetes mellitus: A rising dawn of a new therapeutic concept. J Pak Med Assoc. 2019;69(Suppl 3):S31–5. [PubMed] [Google Scholar]
- 21.Al-Kuraishy HM, Al-Gareeb AI, Hussien NR, Al-Naimi MS, Rasheed HA. Statins an oft-prescribed drug is implicated in peripheral neuropathy: The time to know more. J Pak Med Assoc. 2019;69(Suppl 3):S108–12. [PubMed] [Google Scholar]
- 22.Sheikh MH, Solito E. Annexin A1: Uncovering the many talents of an old protein. Int J Mol Sci. 2018;19:1045–55. doi: 10.3390/ijms19041045. [DOI] [PMC free article] [PubMed] [Google Scholar]


