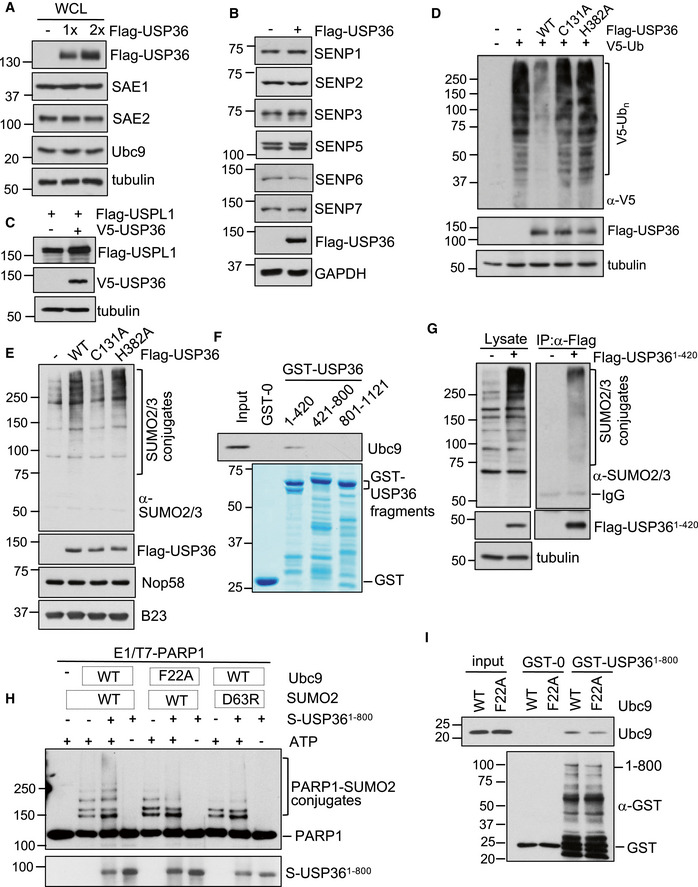
Figure EV2. USP36 neither induces the levels of SUMO E1 or E2 nor increases the levels of SUMO proteases.
-
AUSP36 does not increase the levels of SUMO E1 and E2. H1299 cells transfected with control or increased amounts of Flag‐USP36 were assayed by IB using antibodies against indicated proteins.
-
B, CUSP36 does not reduce the levels of SUMO proteases. H1299 cells transfected with Flag‐USP36 were assayed by IB using antibodies against indicated SENP proteins (B) or with Flag‐USPL1 (C) in the presence or absence of V5‐USP36 were assayed by IB.
-
DH1299 cells transfected with V5‐Ub together with Flag‐USP36 or the indicated mutants were assayed by IB to detect total ubiquitination.
-
EWT USP36 and the H382A mutant, but not the C131A mutant, promote SUMOylation in the nucleolus. H1299 cells transfected with control or the indicated Flag‐USP36 plasmids were subjected to nucleolar isolation, followed by IB. SUMO2/3 conjugates are indicated in the top panel.
-
FThe N‐terminus of USP36 interacts with Ubc9 in vitro. Purified His‐Ubc9 was incubated with GST, GST‐USP361–420, GST‐USP36421–800, or GST‐USP36801–1121. Bound protein was detected by IB. GST and GST‐fusion proteins were shown in the bottom panel by coomassie staining.
-
GThe N‐terminal USP36 binds to SUMO in cells. H1299 cells transfected with either control or Flag‐USP361–420 plasmid were assayed by IP using anti‐Flag, followed by IB with anti‐SUMO2/3 antibodies.
-
HRequirement of the SUMO–Ubc9 backside interaction for USP36's SUMO E3 activity. Recombinant T7‐PARP1 protein (0.1 μM) was incubated with SUMO E1 (50 nM, Boston Biochem), Ubc9 (50 nM, WT or the F22A mutant), SUMO2 (4 μM, WT or the D63R mutant) in the presence of USP361–800 (50 nM) and/or ATP (2.5 mM) at 30°C for 5 h and then assayed by IB.
-
IUSP36 interacts with Ubc9 in vitro. Purified His‐Ubc9 (Wt or the F22A mutant) was incubated with GST or GST‐USP361–800. Bound Ubc9 was detected by IB using anti‐Ubc9 antibody. GST and GST‐fusion proteins were detected by IB with anti‐GST.
Source data are available online for this figure.
