Figure 4. Identification of O‐GlcNAcylation sites of TDP‐43.
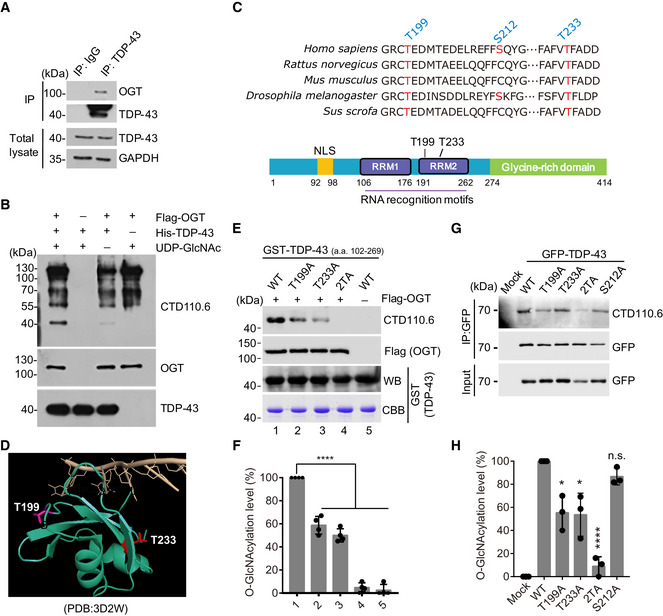
- TDP‐43 interacts with OGT examined by immunoblotting.
- In vitro glycosylation assay was performed using immunoprecipitated OGT incubated with recombinant His‐tagged TDP‐43 in the presence of UDP‐GlcNAc. The O‐GlcNAcylated TDP‐43 protein was detected by immunoblotting.
- Sequence alignment of TDP‐43 segments containing the identified O‐GlcNAcylation sites in different species. The amino acids with red color display the conserved O‐GlcNAcylated sites.
- Structural modeling of the RRM2 domain bound to a single strand DNA illustrates the O‐GlcNAcylation sites T199 (pink) and T233 (red) of TDP‐43 with surrounded DNA (light orange).
- In vitro glycosylation assays were performed using immunoprecipitated Flag‐OGT incubated with recombinant GST‐tagged TDP‐43 fragments (a.a. 102‐269) in the presence of UDP‐GlcNAc. The O‐GlcNAcylated TDP‐43 proteins were detected by immunoblotting.
- Quantification of (E). Mean ± SD, unpaired two‐tailed t‐test, n = 4 biological replicates. ****P < 10−4.
- Cells expressing the indicated forms of GFP‐TDP‐43 were subjected to immunoprecipitation, and the O‐GlcNAcylated levels of TDP‐43 were examined.
- Quantification of (G). Mean ± SD, unpaired two‐tailed t‐test, n = 3 biological replicates. *P < 0.05, ****P < 10−4, n.s. not significant.
Source data are available online for this figure.
