Abstract
Wearable biosensors are garnering substantial interest due to their potential to provide continuous, real-time physiological information in an array of healthcare-related applications via dynamic non-invasive measurements of chemical markers in biofluids, such as sweat, tears, saliva and interstitial fluid (ISF). Recent developments in this area have focused on electrochemical and optical biosensors, with major advances being made in the non-invasive monitoring of new biomarkers, ranging from metabolites to bacteria and hormones. These include the design of multiplexed biosensing approaches and microfluidic sampling/ transport systems, along with system integration and miniaturization combined with flexible materials for enhanced wearability and ease of operation. The increased accuracy, effectiveness and utility of modern wearable biosensing platforms are enhancing both reliability and commercial impact. Even so, our limited understanding of the correlations between analyte concentrations in the blood and non-invasive biofluids remains a major obstacle. In addition, both an expanded set of on-body bioaffinity bioassays and additional sensing strategies will be required to expand the scope and type of biomarkers accessible to monitoring. Further improvements in biosensor accuracy and stability in uncontrolled conditions, along with reproducible sample transport, will be required for improved sensor reliability. Overall, widespread acceptance of wearable biosensors by the medical and commercial communities will require extensive large population validation of their performance through multidisciplinary collaboration between the engineering, biological and clinical disciplines. Overall, real-time body sensing and communication of comprehensive physiological information via wearable biosensing technologies offer significant promise to enhance personal healthcare and performance monitoring with the potential to have a broad impact on our daily lives.
Wearable sensors have received tremendous attention since the arrival of smartphones and other mobile devices, owing to their ability to provide useful insights into the performance and health of individuals1-6. Early efforts in this area focused on physical sensors that monitored mobility and vital signs, such as steps, burned calories, or heart rate. The face of wearable devices has changed rapidly in recent years with researchers branching out from tracking physical exercise activity to focus on tackling major challenges in healthcare applications, such as the management of diabetes or remote monitoring of the elderly. To accomplish these goals, researchers have devoted substantial efforts to the development of wearable biosensors, which are defined as wearable sensing devices that incorporate a biological recognition element into the sensor operation (e.g., enzyme, antibody, cell receptor or organelle). The potential utility of wearable biosensors is evident from the rapidly increasing rate of newly reported proof-of-concept studies. Although several of these platforms are currently under clinical evaluation, successful translation to the commercial market has been lacking. Significant endeavors are currently underway toward the commercialization of non-invasive biosensors. However, these products still require further large-scale validation studies, the necessary regulatory approvals device and final marketing paths. Driven by the promise of the huge glucose sensing market, this commercial activity focuses largely on minimally-invasive glucose monitoring devices, as illustrated in the representative examples given in Table 1.
Table 1.
Selected examples of commercial non-invasive or minimally-invasive biosensors.
| Product/Com pany |
Analyte/Sa mple |
Wearable platform |
Monitoring mechanism |
Current stage | Website |
|---|---|---|---|---|---|
| Google/Norvatis | Glucose in tears | Contact lens | Electrochemistry | Last update was made in 2014, discussed with FDA toward clinical trial | https://verily.com/projects/sensors/smart-lens-program/ |
| Glucowatch/Cygnus | Glucose in ISF | Watch type | Electrochemistry | FDA approved, but retracted from market | No longer available |
| BioMKR/Prediktor Medical | Blood glucose | Wrist strap type similar to smartwatch | Near IR spectroscopy, bio-impedance | Currently running clinical testing for approval and market launch in Europe | https://www.prediktormedical.com/ |
| Glucowise/MediWise | Blood glucose | Finger clip type | Radiowaves | Under development, running clinical trials with healthy volunteers | http://www.gluco-wise.com/ |
| Freestyle Libre/Abbott | Glucose in ISF | Patch type | Electrochemistry | FDA approved in US on July 2018. | https://www.freestylelibre.us/ |
| Dexcom G6 CGM/Dexcom | Glucose in ISF | Patch type | Electrochemistry | FDA approved | https://www.dexcom.com/ |
| GlucoTrack/Integrity Applications | Blood glucose | Finger clip type | Ultrasonic, electromagnetic, and thermal waves | Type 2 diabetes, currently approved in Europe | http://www.glucotrack.com/ |
| Eversense/Senseonics | Subcutaneous implant for ISF glucose | Small stick type | Using fluorescence | Recently received FDA approval | https://www.eversensediabetes.com/ |
| Noviosense | Placed under the lower eyelid, tear glucose | Small stick type (spiral type) | Electrochemistry | Tested in animals and human subjects | http://noviosense.com/ |
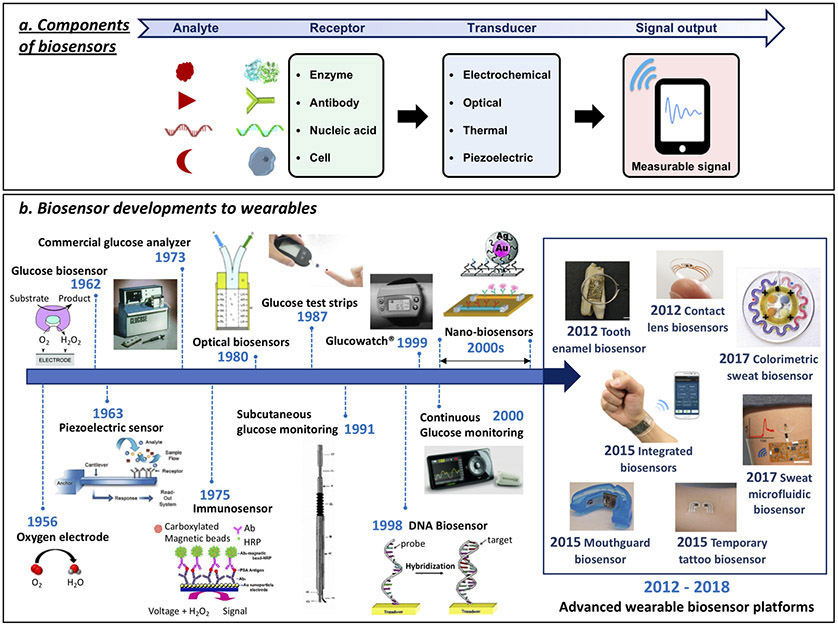
A typical biosensor contains two basic functional units: a ‘bioreceptor’ (e.g., enzyme, antibody or DNA) responsible for selective recognition of the target analyte, and a physico-chemical transducer (e.g., electrochemical, optical or mechanical) that translates this biorecognition event into a useful signal (Fig. 1a). Such devices were initially developed for in vitro measurements in controlled (laboratory or point-of-care) settings or for single-use home testing (e.g., blood glucose test strips). A brief history of biosensing technologies preceding current wearable biosensors is provided in Fig. 1b7-38. These past advances have paved the way to modern wearable biosensors for non-invasive biomonitoring applications as an alternative to blood monitoring biomedical devices, in connection to wide range of healthcare applications.
Figure 1.
Biosensor components and the path of biosensor development for wearables. (a) Schematic representation of biosensor operation principles: Target analyte detection by corresponding receptor molecule followed by signal transduction method and output. (b) The concept of enzyme electrodes was proposed by Clark and Lyons in 19627. Their device relied on entrapment of the enzyme glucose oxidase (GOx) over an amperometric oxygen electrode that monitored the oxygen consumed by the biocatalytic reaction. Clark’s electrochemical biosensor technology was transferred to the Yellow Spring Instrument (YSI) Company, which launched the first dedicated blood glucose analyzer (YSI Model 23 Analyzer) in 1975. Biosensors became a ‘hot’ topic during the 1980s, reflecting the growing emphasis on biotech. New biosensor transduction principles were introduced during this decade, including fiber-optic and mass-sensitive (piezoelectric) devices8-14. Considerable efforts during the 1980s led also to the introduction of commercial self-testing blood glucose strips that used mediator-based enzyme electrodes15,16. Subsequent activity during the 1990s resulted in subcutaneously implantable needle-type electrodes for real-time in vivo glucose monitoring17. These subcutaneously implantable glucose sensors moved in the early 2000 to commercial continuous glucose monitors (CGMs) that track in real-time the glucose level in the ISF, along with diabetes relevant trends and patterns18,19. The emergence of nanotechnology in the late 1990s has led to variety of nanomaterial-based biosensors exploiting the attractive properties of different nanomaterials, such as silicon nanowires and gold nanoparticles, for label-free or amplified biosensing, respectively20,21. The specific base-pair recognition of DNA sequences led to the development of different DNA biosensors in the late 1990s22-24. Such nucleic acid sensors are currently playing a growing role in genomic sequence analysis. These advances in biosensor technology over the past five decades paved the way to modern wearable biosensors, discussed in this article. (Glucose biosensor adapted from J.W. et al.25). Piezoelectric sensor adapted from ref. 26. Commercial Glucose Analyzer adapted from ref. 27. Immunosensor adapted from ref. 28. Optical Biosensor adapted from ref. 10. Glucose test strips adapted from ref. 29. Subcutaneous glucose monitoring adapted from ref. 17. GlucoWatch adapted from ref. 30. DNA Biosensor adapted from ref. 24. Continuous glucose monitoring adapted from ref. 31. Top nanobiosensors adapted from ref. 21. Bottom nanobiosensors adapted from ref. 20. Tooth enamel biosensor adapted from ref. 32. Contact lens sensors adapted from ref.33. Colorimetric sweat biosensor adapted from ref. 34. Integrated biosensors adapted from ref. 35. Mouthguard biosensor adapted from (J.K., J.W et al.)36. Temporary tattoo biosensor adapted from J.W. and colleagues37. Sweat microfluidic sensor adapted from (J.K., A.S.C., J.W. et al.)38.
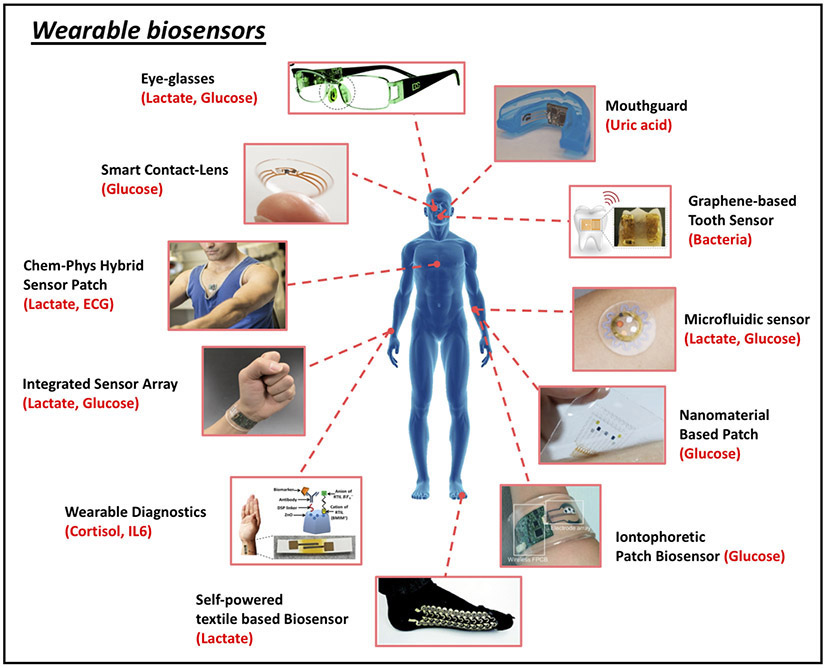
Biosensors hold considerable promise for wearable applications due to their high specificity, speed, portability, low cost and low power requirements. Indeed, innovative biosensor platforms for non-invasive chemical analysis of biofluids, such as sweat, tears, saliva or interstitial fluid (ISF), have already been widely applied to a variety of head-to-toe, on-body application sites, targeting an array of important analytes in proof-of-concept demonstrations (Fig. 2)32-36,39-44. Sweat, tears, saliva and ISF have been targeted as they can be sampled in a non-invasive manner, meaning that they can be readily accessed without disrupting the outermost protecting layers of the body’s skin (i.e., stratum corneum) and without contacting blood. As such, non-invasive sensing methods pose minimal risk of harm or infection and are generally more user-friendly.
Figure 2.
Representative examples of wearable biosensors. (clockwise from top): Eyeglasses-based wireless electrolyte and metabolite sweat sensor (adapted from J.W. et al.39). Wearable salivary uric acid mouthguard-based biosensor (adapted from J.K., J.W. et al.36). Graphene-based wireless bacteria sensor applied on tooth enamel (adapted from ref. 32). Wearable microfluidic sweat sampling device for colorimetric sensing of sweat (adapted from ref. 34). Graphene-based sweat sensor with thermoresponsive microneedles for diabetes monitoring and therapy (adapted from ref. 40). Integrated wearable sensor arrays for multiplexed sweat extraction and analysis (adapted from ref. 41). Stretchable self-powered sweat biosensors on textile platform (adapted from J.W. et al.42). Sweat-based wearable diagnostics biosensors using room-temperature ionic liquids (adapted from ref.43). Integrated multiplexed wearable sensor arrays for in situ perspiration analysis (adapted from ref. 35). Wearable chemical-electrophysiological (lactate/ECG) hybrid biosensor for real-time health and fitness monitoring (adapted from J.W. et al.44. Smart contact-lens biosensing platform for glucose monitoring in tears (adapted from ref. 33).
The wide acceptance of such wearable biosensor technology requires a deep understanding of the biochemical composition of bodily fluids, such as sweat or tears, and its relation to blood chemistry. Wearable monitoring platforms can shed useful insights into dynamic biochemical processes in these biofluids by enabling continuous, real-time monitoring of biomarkers, which can be related to a wearer’s health and performance. Such real-time monitoring can provide information on wellness and health, enhance the management of chronic diseases and alert the user or medical professionals of abnormal or unforeseen situations. Wearable biosensors can obviate painful and risky blood sampling procedures and can be readily blended with a wearer’s daily routine. To accomplish this capability, the biosensing platform must provide direct contact with the sampled biofluids without inducing any discomfort to the wearer. Such body compliance can be achieved through use of advanced materials and smart designs that provide the necessary flexibility and stretchability45-48. Continuous multidisciplinary development of new biosensing technologies (and corresponding new materials and energy sources) has led to numerous proof-of concept demonstrations and has driven growing efforts towards the commercialization activity of wearable sensors.
The attractive capabilities of modern wearable chemical and physical sensors and related research advances have been highlighted in several recent reviews2, 29, 48-53. Unlike physical or chemical wearable sensors, the wearable biosensors reviewed here rely on highly specific bioreceptors capable of recognizing target analytes in complex samples at their physiologically-relevant concentrations. Despite rapid progress in wearable biosensor technology over the past 5 years, we are only at the beginning of understanding how wearable biosensor technologies can improve health and performance.
In the following Review, we provide an overview of the key advances in wearable biosensors from the past 2 years and discuss their potential as alternatives to invasive biomedical devices and to gold standard blood assays. In particular, we discuss how the fundamental principles of biosensor systems can be adapted to the design of reliable wearable biosensors, we highlight key challenges in operating biosensors in specific non-invasive biofluids and the physiological relevance of monitoring key biomarkers in these fluids, and finally we provide an overview of the overall importance and future prospects of wearable biosensing devices for the biomedical field. Pioneering studies that greatly impacted the field of wearable biosensing are critically reviewed along with future challenges to overcome. The majority of the studies discussed here involve biosensing devices based on electrochemical signal transduction along with some optical-sensing devices, as these transduction mechanisms have been the most commonly reported on over the past several years. Emphasis is given to systems aiming at practical healthcare applications with promise for clinical translation in the near future.
The commercialization of wearable bioanalyte sensors is substantially more challenging than that of activity-tracking counterparts or common lab-based biosensors because such devices must be capable not only of continuous on-body biochemical sensing but also of reliable measurement of a biorecognition element (or elements) that is highly specific yet fragile. Robust, reliable measurement also must overcome such challenges as gradual surface biofouling at the body–sensor interface, inefficient transport of sample over the sensor, limited stability of many bioreceptors, the complexity of multi-step bioaffinity assays and related receptor regeneration, and issues posed by calibration for on-body biosensors. In each section below, we discuss specific challenges related to each particular system and biofluid. Finally, we discuss future research and commercialization prospects, existing bottlenecks and present our perspective on the prospects for this exciting research area.
Epidermal wearable biosensors
Taking into account that the epidermis covers an overwhelming majority of our body, skin-worn conformal devices have received the greatest recent attention among the various types of wearable biosensors. Epidermal biosensors can facilitate real-time analysis of biomarkers in epidermal biofluids (sweat and ISF) with some systems exhibiting continuous monitoring capabilities toward a variety of biomedical and fitness applications. These devices rely on sweat or ISF sampling at the skin surface, along with transport of these biofluids over the biosensor surface. Such skin-worn biosensors commonly rely on different transduction modes (e.g., optical, electrochemical, and mechanical) along with biocatalytic and ion-recognition receptors. Further integration with data processing and transmission components are necessary for a fully wearable platform. The majority of recent reports, however, have focused on electrochemical and colorimetric transduction methodologies. Major progress has been made toward a variety of skin-worn platforms offering the capability to readily sample epidermal biofluids along with wearer comfort3, 34, 35, 40, 42, 50, 54-63. Such devices have been realized through direct transfer of sensors onto the skin (using E-skin or printed temporary tattoos), by sensor incorporation into wrist-bands and patches, or by embedding sensors directly into textiles to ensure tight contact with the skin while allowing endurance of mechanical stresses encountered during body movements.
Secretion and composition of epidermal biofluids (sweat, ISF).
Sweat is the most readily obtainable biofluid for chemical sensing applications since sweat glands are distributed across the entire body with more than 100 glands/cm2 of skin. This physiology provides the most viable sampling sites and surface area outside the body. However, sweat must be excreted to the outer skin surface to be analyzed. Such sweat generation can be accomplished through exercise activity, thermal heating, stress, or iontophoretic stimulation. Generally, sweat contains metabolites (e.g., lactate, glucose, urea, ethanol or cortisol) along with electrolytes (e.g., sodium, potassium, chloride or ammonium), trace elements (e.g., zinc or copper), and small amounts of large molecules (e.g., proteins, nucleic acids, neuropeptides or cytokines)64. These biomarkers make in situ sweat analysis of considerable interest for non-invasive monitoring of physiological health status (e.g., hydration or physical stress) and for disease diagnosis and management (e.g., in such conditions as cystic fibrosis or diabetes). Non-invasive monitoring at the epidermis eliminates issues related with blood sampling while maintaining the protective stratum corneum skin layer intact. Yet, additional research is needed for determining and validating the clinical value of sweat as a diagnostic biofluid. Target sweat analytes are each transported to the sweat from surrounding capillaries with unique partitioning profiles, making reliable correlation to concurrent blood concentrations difficult. Analytes can reach the sweat by passive (i.e., diffusion) or active mechanisms and can be also generated within the sweat duct itself. Although variations in sweat rate can be monitored using multiplexed analysis (i.e., simultaneous monitoring of analytes with concentration profiles that are independent of sweat rate) or skin impedance measurements, the degree of analyte dilution during sweat excretion is affected by the relationship between sweat rate and analyte partitioning rate64. Deeper understandings of sweat chemistry and transport, along with advances in sweat sampling and detection technologies, should accelerate sweat-based diagnostic opportunities.
Alternatively, epidermal biosensing systems have targeted measurements of analyte concentrations in ISF. Within the viable skin tissue, skin cells are surrounded by ISF, which provides nutrients that diffuse directly from the capillary endothelium. This function, and the associated ISF composition, lead to reliable correlations between the blood and ISF concentrations of many analytes, including electrolytes (e.g., sodium, phosphate, magnesium, potassium or calcium), metabolites (e.g., glucose, alcohol, lactate or cortisol) and proteins65-69. However, to evaluate ISF analytes in a non-invasive manner, these components must be extracted to the skin surface, which can be accomplished through reverse iontophoresis (RI) or sonophoresis. Using these methods, variations in the extraction efficiency and skin surface contamination can impact the accuracy, similar to sweat-based platforms. To address such issues, advanced sampling methodologies and refinement of each analyte monitoring approach is necessary.
Exercise-based wearable sweat biosensors.
Early advances in epidermal wearable biosensing platforms focused on single analyte sensing with a wide range of targeted analytes (A.S.C., J.K., J.W et al.) 1, 29, 49, 50, 66. Such proof-of-concept demonstrations were made using new stress-enduring materials and sensor structures for achieving the high degree of skin conformability essential for reliable sweat sampling during exercise, such as tattoo-type platforms. Temporary tattoos, coupled with screen-printed flexible electrodes, offer an attractive platform for skin-worn biosensing devices as they allow direct and continuous contact with the skin surface (J.W. et al.)50. Such body-compliant sensors couple highly favorable substrate skin elasticity and tight contact with the skin, with an attractive electrochemical performance. Tattoo-based epidermal biosensors have thus been shown to allow real-time, non-invasive measurements of key sweat electrolytes (pH, ammonium or sodium), heavy metal (Zn) and metabolites (lactate or ethanol) (J.K., J.W. et al.)58,70-74. For example, our group (J.W. et al.)58 published the first demonstration of continuous monitoring of sweat lactate levels via epidermal electrochemical biosensors, providing a real-time profile of the lactate sweat dynamics during exercise. Sweat lactate is a byproduct of local sweat gland metabolism and intense physical activity induces higher generation rates. Although sweat lactate does not directly reflect the concurrent blood levels, it indicates the level of physical exertion experienced during prolonged exercise and can be used as a marker for athletic efficiency without invasive blood sampling. In this study58, the human subject was asked to wear the printed temporary tattoo biosensor, modified with lactate oxidase (LOx), for assessing the sweat lactate level during exercise, which indeed increased with higher exercise intensity.
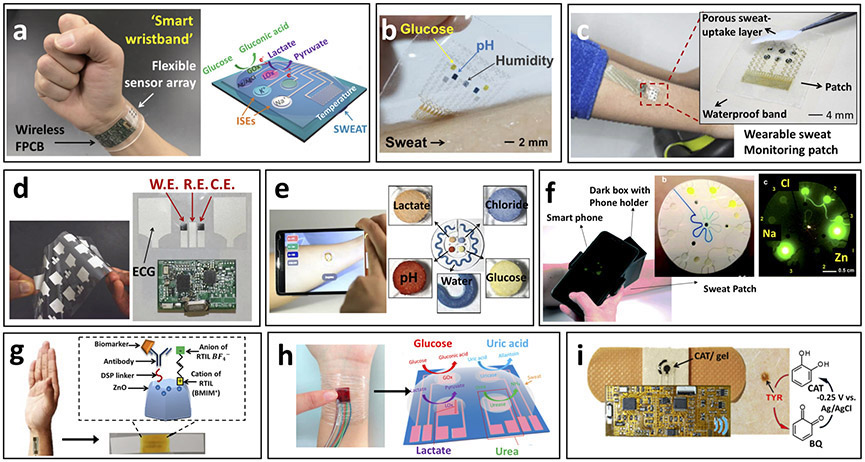
A tremendous advance has been made in developing multiplexed sweat biosensor platforms for quantitative analysis of sweat based on a fully-integrated patch-based wearable sensor array (Fig. 3a)35. Given that sweat is a bio-information rich and non-invasively accessible biofluid, simultaneous non-invasive multianalyte sensing is extremely attractive but requires an accurate monitoring system. In this work, the Berkeley team demonstrated the simultaneous multiplexed detection of sweat metabolites (glucose and lactate) and electrolytes (sodium and potassium ions), along with skin temperature by integrating a multi-sensing array. This pioneering work greatly advanced the wearable sensing field by filling the gap between signal transduction, conditioning, data processing, wireless transmission and system integration, allowing in situ data processing and communication. This advance was accomplished by merging flexible patch-type sensors with a conformal circuit board for accurate assessment via advanced signal processing of the physiological states of the human subjects during prolonged exercise. Recently, multi-analyte electrochemical sensing technology was demonstrated by weaving multiple sensing fibers into a soft fabric75. The glucose, Na+, K+ Ca2+, and pH sensing fibers, prepared by coating the recognition materials onto carbon nanotube (CNT) fibers to form a coaxial structure, maintained their attractive real-time sensing performance under repeated deformations. Reliable in situ multi-analyte monitoring is essential for allowing greater personalized diagnostic and physiological monitoring capabilities in a single wearable device. Multianalyte sensing could also provide a measure of sweat rate for calibrating the target analyte signals toward improved physiological relevance. The reported system proved advantageous for monitoring fitness parameters during exercise, but its utility would be limited in continuous monitoring applications due to its reliance on physical exertion for sweat generation.
Figure 3.
Epidermal biosensors for real-time monitoring of sweat chemistry. (a) Depiction of integrated wearable sensor arrays for multiplexed perspiration analysis applied to wrist with schematic representation of sensing array configuration. Fully integrated multianalyte sensor array for sweat-based monitoring of glucose, lactate, sodium, potassium and temperature during exercise with wearable platform containing sensing array as well as signal transduction, conditioning, processing and transmission components (adapted from ref. 35). (b) Depiction of graphene-based sweat sensor array for diabetes monitoring applied to human forearm. Multiplexed patch-type sensor array used for glucose monitoring during exercise with simultaneous measurement of pH, temperature and humidity for glucose signal correction (adapted from ref. 40). (c) Depiction of wearable sweat monitoring patch for sweat-based glucose monitoring and therapy applied to human forearm during exercise. Inset: sweat-based glucose monitoring sensor array configuration with porous sweat-uptake layer. Multiplexed glucose monitoring patch capable of operating in low sweat volumes (adapted from ref. 78). (d) Depiction of wearable chemical-electrophysiological hybrid biosensor configuration for real-time health and fitness monitoring with example of screen-printed electrodes. Simultaneous monitoring of sweat lactate levels and heart-rate for athletic performance evaluation (adapted from J.W. et al.44). (e) Depiction of colorimetric microfluidic sweat sampling device configuration for chemical analysis of sweat with representation of sweat-filled device and smartphone-based signal analysis. Device exhibited enhanced microfluidic sampling of sweat during exercise with wireless quantitative measure of target pH, lactate, glucose and chloride (adapted from ref. 34). (f) Depiction of fluorometric skin-interfaced microfluidic platform for the measurement of chloride, sodium and zinc in exercise induced sweat. Fluorescent probes selectively react with target biomarkers upon sweat flow through the microfluidic system with fluorescent intensity analyzed via smartphone-based imaging module, which obviates the need for electrochemical or colorimetric analyses (adapted from ref. 84). (g) Schematic representation of wearable diagnostic antibody-based biosensor targeting detection of IL-6 and cortisol in human sweat using room temperature ionic liquids for enhanced antibody operational stability. Biosensor configuration with antibody immobilization is shown with depiction of device application onto a human forearm. This device exhibited prolonged stability in pooled human sweat with continuous combinatorial analyte detection within the physiologically relevant concentration range (adapted from ref. 43). (h) Schematic representation of self-powered multifunctional electronic skin used for continuous monitoring of lactate, glucose, uric acid, and urea in exercise-induced sweat using piezoelectric-linked enzymatic biosensors. During exercise, this device was capable of monitoring these biomarkers related to personal health status without an additional power supply through piezoelectric-enzymatic-reaction coupling (adapted from ref. 56). (i) Depiction of wearable tyrosinase sensing bandage for non-invasive melanoma screening. Inset: schematic representation of tyrosinase detection paradigm. Bandage-type wearable sensor for portable cancer biomarker detection (adapted from (J.W. et al.86).
In another example of the advantages of multiplexed wearable devices, notable advances were demonstrated through sweat glucose monitoring devices coupled with pH, humidity, and temperature sensors and integrated with a transdermal drug delivery system (Fig. 3b)40. The sweat glucose biosensor was thus coupled with therapeutic applications toward the management of diabetes. The accurate measurement of physiologically relevant sweat glucose concentrations with epidermal biosensors faces several major challenges related to uncontrolled operational conditions (e.g., varying temperature and pH), glucose contamination from various sources, irregular sampling rates, and low sampling volumes. Although several studies have indicated that sweat glucose concentrations can correlate with concurrent blood levels, these limitations can significantly impact the accuracy of collected data 64, 76,77. In an initial report, functionalized graphene was introduced to stretchable serpentine-structured electrodes for enhanced electrochemical biosensing of sweat glucose during physical exercise. The multiplexed sensing design allowed for continuous correction of the measured results by addressing variations in the activity of the immobilized enzyme caused by pH, temperature and humidity fluctuations, towards enhanced operational accuracy. However, sample contamination by external glucose sources (e.g., glucose from the skin surface, environment or old sample) must yet be accounted for. The developed platform was able to monitor fluctuations in human sweat glucose levels over a day and was further integrated into a closed-loop system utilizing polymeric microneedles for delivering the drug metformin for regulating glucose in a mouse model. The successful combination of transdermal glucose detection with a drug delivery platform represents a significant advance toward reliable ‘Sense-Act’ systems. In further work, the system was improved for efficient sweat control and sensing accuracy by modifying the device assembly with multiple sweat-uptake and waterproof layers, and by miniaturizing the sensor to enable reliable measurements using ~1 μL sweat volumes (Fig. 3c)78. By overcoming low sample volume limitations, these improvements targeted several other challenges of epidermal biosensors. However, efficient sample transport and reliable response would require to consistently provide fresh sample and account for fluctuations in the sweat rate. The advances reported in these studies illustrate the potential of patch-type sweat biosensors for regulating glucose levels. Yet, these devices require physical exercise for their operation, and are thus not compatible with continuous glucose monitoring in daily-life without exercise. Successful implementation of such sweat monitoring devices for managing diabetes would further require extensive large-scale population validation studies.
Our group (J.W. et al.)44 has also reported a new approach to multiplexed wearable sensing that fuses electrophysiological measurements with assays of biochemical markers. This method offers more comprehensive fitness monitoring by simultaneous measuring of physiochemistry (sweat lactate) and electrophysiology (electrocardiogram) than separate physical and chemical sensors. This idea was realized by developing a screen-printed hybrid Chem-Phys patch-type sensor (Fig. 3d)44. On-body experiments involving a stationary cycle revealed that lactate and heart rate can be monitored simultaneously without cross-talk, representing an important first step toward multi-modal wearable sensors for comprehensive understanding of human physiology. Such sensing platforms can provide enhanced monitoring of athlete performance during exercise but will require attention to potential variations in the sweat rate, by applying multiplexed sensing of different parameters for fitness/healthcare monitoring application, similar to previously discussed systems35,40,41,78.
In addition to electrochemical detection techniques, colorimetric signal transduction has been exploited, taking advantage of its ability to monitor target analytes in sweat in connection to different indicator dyes34, 56, 60, 79-82. Colorimetric analysis obviates the need for powering of the sensor platform, which can allow small and readily wearable devices, but requires additional read-out devices with data analysis for sensitive measurements, such as a camera with color analyzing software. Real-time optical monitoring of multiple sweat biomarkers is accomplished using a colorimetric sensing system integrated with microfluidics for real-time sweat sampling. The developed device allows sophisticated sweat sampling and measurement based on a thin and soft closed microfluidic system that directly and rapidly collects the generated sweat without sweat evaporation or contamination, resolving the conventional challenges of sweat (Fig. 3e)34. Such skin-mounted fluidic devices were designed to monitor multiple sweat biomarkers (e.g., lactate, glucose, pH, chloride or sweat loss) through multiple channels and corresponding sensing reservoirs along with quantification of sweat loss. The colorimetric data obtained with two human trials were analyzed and quantified through wireless data transmission. The Rogers group83 further refined their epidermal, colorimetric sweat sensing microfluidic platform via super absorbent polymer valves that provide the capture and storage of generated sweat for analysis of chloride concentrations toward multiple sequential measurements. Our group (J.K., A.S.C., J.W. et al.)38 has also recently developed an analogous skin-worn flexible sweat sampling microfluidic flow system, with integrated electrochemical biosensing of lactate and glucose.
The latest advance in such microfluidic sweat monitoring technologies has been accomplished by incorporating fluorescent probes into a skin-interfaced system for accurate in situ measurement of chloride, sodium and zinc with the resulting fluorescence evaluated via smartphone-based imaging module (Fig. 3f)84. This optical sensing fluidic approach offers sensitivity comparable to conventional laboratory techniques with operation in microliter volumes. Such expansion of viable signal generation and transduction methodologies is crucial for broadening the scope of targetable biomarkers, particularly when successfully coupled to biofluid sampling methods that do not necessitate exercise.
Several recent studies have also focused on the expansion of target biomarkers to include those related to hormone and immune responses. For instance, a wearable immunosensor for detecting cortisol and interleukin 6 (IL-6) in sweat has diagnostic potential (Fig. 3g)43. This platform was evaluated in vitro with human sweat, using room-temperature ionic liquids to compensate for variations in sweat pH while enhancing the stability of the antibody receptor for up to 96 hours. Furthermore, label-free electrochemical impedance spectroscopy was applied to detect the analyte-binding event while porous polyamide membranes were used for effective sweat sampling in low volumes for application on the human finger or hand. Similarly, an alternative cortisol detection system has been reported based on MoS2 nanosheets functionalized with cortisol antibodies85. Although such antibody-based bioassays hold great promise for expanding the scope of epidermal wearable biosensors, which focus primarily on enzymatic metabolite detection, their successful on-body evaluation has yet to be demonstrated. Unlike epidermal enzyme-based biosensors, such immunosensors cannot be readily regenerated toward continuous monitoring applications, and along with other challenges of multi-step affinity bioassays, these devices require further efforts and innovations.
Most recent progress in wearable biosensors has been made using electrochemical or optical methods, but piezoelectric biosensing systems have also been introduced as new electronic-skin platforms monitoring sweat metabolites (Fig. 3h)56. The resulting piezoelectric signal is driven by the body movement (during exercise) and depends on the analyte sweat concentration. This results in a self-powered biosensor capable of distinguishing sweat analyte concentrations and obviating the necessity of power supply or battery. The validation of such proof-concept of wearable piezoelectric biosensors as self-powered device in real-world applications requires critical evaluation in terms of accuracy and duration of use.
Epidermal biosensors can also analyze the skin surface rather than detecting sweat or ISF biomarkers. Unlike earlier wearable biocatalytic sensors designed for detecting the corresponding metabolite substrates, a recently reported bandage-type biosensor was proven capable of detecting the enzyme tyrosinase on the skin surface as an analyte (Fig. 3i; J.W. et al.86). This system represents the first example of a wearable device aimed at detecting an enzyme as a biomarker. Selective tyrosinase detection was accomplished by immobilizing catechol, the substrate of tyrosinase enzyme onto the sensor surface. The tyrosinase level was thus determined electrochemically by measuring the benzoquinone, product of the enzymatic reaction. The attractive performance of this new tyrosinase bandage biosensor indicates promise for rapid screening of melanoma. Bandage-type wearable sensors represent a rapidly emerging technology with considerable potential for low-cost decentralized (home or point-of-care) monitoring and diagnoses. Each system, however, still requires extensive on-body validation and human testing of clinical accuracy.
Iontophoresis-based epidermal biosensors.
Epidermal biofluids (ISF and sweat) can also be non-invasively obtained through iontophoresis in connection to important bio-monitoring uses. This method involves the application of a mild current across the skin to induce ion migration between two skin-worn electrodes and can be accomplished at rest. Iontophoresis is a non-invasive method of transporting molecules through the skin without harming the skin surface or contacting blood. ISF can be extracted through RI, which relies on application of a low current to induce a flux of positively charge ions toward the negatively charged skin surface and an electro-osmotic flow from the anode to cathode. This flow further results in the movement of neutral molecules, such as glucose, toward the cathode. ISF glucose levels display good correlation with blood glucose since ISF components diffuse directly from the capillary endothelium. The extracted glucose in ISF can be easily measured using glucose biosensors mounted on the skin. The first commercial demonstration of a RI-based sensing platform was developed by Cygnus as a wearable, wrist-mounted system called the GlucoWatch Biographer30. This US Food and Drug Administration (FDA)-approved device is capable of non-invasive glucose monitoring over a 12 hour period with 6 measurements per hour. However, the GlucoWatch was withdrawn from the market in the early 2000s due to reported skin irritation caused by the RI process, long warm-up period (2–3 h) and the necessity of calibration using an invasive blood glucose meter. This example indicates that commercialization of wearable biosensors requires careful evaluation in terms of accuracy and ease of use.
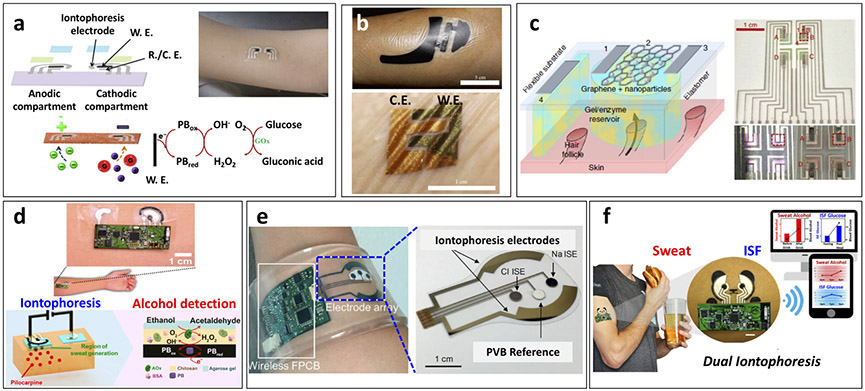
Later, work in our group (J.W. et al.)37 resulted in the development of an iontophoresis-based platform using a body-compliant flexible tattoo platform through screen-printing both the iontophoretic electrodes for RI and the glucose biosensing electrodes (Fig. 4a). This integrated device addressed several limitations of the Glucowatch: first, it minimized discomfort during RI by reducing the applied iontophoresis current and glucose detection potential; second, it greatly reduced the price by relying on a disposable screen-printed tattoo platform; and third, it was easily mounted on the skin surface without hindrance to the wearer’s movement. The performance of the tattoo sensors was evaluated in healthy human subjects by comparing the response obtained before and after meal, and then validating the results with concurrent blood glucose test strips. This proof-of-concept demonstration highlighted the capability of disposable tattoo-based wearable glucose sensing platforms to use RI for ISF sampling, but lacked electronics integration and validation of long-term operation toward continuous monitoring applications.
Figure 4.
Epidermal iontophoretic biosensors. (a) Schematic representation of epidermal reverse iontophoretic tattoo-based glucose sensor configuration and operation paradigm with on-body depiction of device applied to human subject. Proof-of-concept demonstration of reverse iontophoretic tattoo-based ISF glucose sensor (adapted from J.W. et al.37). (b) On-body depiction of iontophoretic paper battery and skin-like biosensor for non-invasive blood glucose monitoring applied to human subject. Inclusion of hyaluronic acid facilitated enhanced ISF extraction for increased ISF glucose sampling reliability (adapted from ref. 87). (c) Schematic representation of transdermal, path-selective iontophoretic ISF sampling approach using miniaturized graphene-based pixel arrays for non-invasive glucose monitoring. Configuration of pixel-type biosensor array with four individual ISF extraction and detection locations. This proof-of-concept device exhibited the capability to sample ISF through individual follicular pathways for enhanced glucose detection reliability over 6 hours by focusing on device architecture design rather than specific sensor implementation (adapted from ref. 88). (d) Depiction of epidermal iontophoretic alcohol sensing tattoo applied to human subject with schematic representation of iontophoretic drug delivery and sensing paradigms. Localized, drug-induced sweat generation for on-demand sampling of sweat alcohol at a patch-type sensor platform (adapted from J.K., J.W. et al.74). (e) On-body depiction of integrated wearable sensor array band for multiplexed sweat extraction and analysis applied to human wrist with schematic representation of sensor array configuration. Simultaneous detection of chloride, sodium and glucose in iontophoretic induced sweat (adapted from ref. 41). (f) Device configuration and on-body application of simultaneous dual iontophoretic ISF and sweat sampling platform for the sampling and analysis of these two bio-fluids on a single platform without cross-contamination. This device demonstrated the capability to monitor sweat alcohol and ISF glucose simultaneously through the iontophoretic delivery of sweat inducing pilocarpine and iontophoretic extraction of ISF, which were shown to correlate to concurrent trends in blood concentrations (adapted from J.K., A.S.C., J.W. et al.93).
To enhance the collection of ISF glucose, the delivery of positively charged hyaluronic acid was incorporated into wearable platforms, which led to increased transport of glucose to the skin surface87. The extracted ISF glucose was measured by a conformal GOx-based biosensor attached at the site of ISF extraction after the RI process (Fig. 4b). This approach increased the ISF glucose sampling efficiency for more accurate sensing in relation to blood concentrations, indicating promise for enhanced non-invasive RI-based monitoring applications that address the limitations of the GlucoWatch. These RI-based glucose sensing devices take advantage of the close correlations between ISF and blood glucose levels as well as the capability of RI to sample ISF at rest. However, the efficiency of glucose extraction by RI is difficult to control, which can lead to inconsistent volumes of sampled ISF and thus variations in glucose concentration.
Toward greater consistency of RI analyte extraction, a path-selective, graphene pixel-based glucose monitoring patch was recently developed (Fig. 4c)88. This platform applied an array of small ‘pixels’ designed to be roughly the size required to sample ISF from a single hair follicle as the follicular path was shown to be the preferential, low resistance path of ISF extraction, and thus provide for greater extraction reproducibility. Arrays of multiple pixels allowed for redundant measurements to be taken on a single platform for greater accuracy. Such reliable operation could prove crucial to the successful implementation of epidermal wearable biosensors. Successful on-body non-invasive glucose monitoring was demonstrated for over 6 hours. Extended operation is yet a major requirement for clinical translation and commercial viability.
Iontophoresis has also been widely used recently to stimulate local sweat secretion by loading the iontophoretic electrodes with a sweat stimulant (e.g., pilocarpine and carbachol). Using this method, sweat generation can be controlled on-demand, obviating the need for exercise and enabling measurement at rest. Iontophoresis was developed in 1959 by Gibson and Cooke89, who introduced the use of pilocarpine for sweat generation. The cationic drug pilocarpine can be delivered across the skin through charge repulsion at the anode compartment, leading to localized sweat production. This process was further developed as the commercial chloride ion monitoring product Macroduct by Wescor toward the FDA-approved diagnosis of cystic fibrosis90,91. A recent paper from our group (J.K., J.W. et al.)75 describes the merging of an iontophoretic sweat-generation system with an amperometric biosensing system in a single wearable tattoo platform (Fig. 4d). This integrated tattoo biosensor measured sweat alcohol in the ionotophoretically generated sweat within 10 min. Sweat alcohol represents a useful indicator for blood alcohol levels without time lag and errors common to transdermal devices and breathalyzers. Highly selective alcohol measurements were achieved by coupling the alcohol-oxidase enzymatic reaction with the cathodic detection of the liberated peroxide product at the printed Prussian Blue transducer. Wireless bluetooth interface enabled signal transmission to a mobile device. The analytical performance was demonstrated and validated with healthy human subjects consuming different levels of a variety of alcoholic beverages. Iontophoresis-induced sweat generation obviates the need to exercise for sweat sampling. Yet, due to potential variation in the sweat rates active calibration is required to ensure reliable measurement.
Other efforts have focused on integrating the iontophoretic sweat-generation compartment and sensing compartment on different skin mountable platforms. For example, using agonist delivery to generate sweat, a patch-type iontophoretic sweat sensor has been developed either for measuring sodium and chloride ions in cystic fibrosis diagnosis or for measuring glucose concentrations in healthy individuals (Fig. 4e)41. Specific sweat-generating profiles can be generated in response to stimulation with pilocarpine or alternative agonist agents, such as acetylcholine and methacholine. Device performance was evaluated by comparing the electrolyte data of healthy subjects and cystic fibrosis patients; in addition, the ability of the sensor to detect glucose was assessed in the context of monitoring sugar consumption in healthy subjects. This advanced device was capable of non-invasively monitoring target biomarkers in a fully integrated platform with tailorable sweat generation profiles. However, the duration of sweat generation was limited to 60 min, with varying rates over that time, which could hinder continuous monitoring applications.
Future development of epidermal wearable biosensors should also expand their utility to the detection of various drugs toward non-invasive pharmacokinetic studies. For example, a wearable sweat-based sensor has been developed for detecting caffeine (a methylxanthine drug) using pilocarpine-based iontophoretic sweat stimulation or exercise-induced sweat92. Rather than using biological recognition, this sensing platform relied on direct anodic detection of caffeine at a carbon nanotube-based working electrode with a voltammetric scan. Although this device is not classified as a typical biosensor, the proof of principle experiments indicate the potential of such sensing systems for monitoring drug substances and drug interactions in the human body, with the promise of theranostic (therapy plus diagnostic) applications farther into the future. Even so, as described, the sensing device was not integrated with the sweat generation device, but rather coupled to a commercially available Macroduct sweat collector using pilocarpine delivery. For widespread pharmacokinetics studies performed at rest, a customized iontophoretic device should be integrated with the sensing platform. Additionally, a deeper understanding of blood–sweat drug concentration correlations will be required to facilitate meaningful data collection and interpretation.
Despite the tremendous advances in epidermal biosensors, the reported devices have been limited to analysis of a single sampled biofluid. However, our group (J.K., A.S.C., J.W. et al.)93 recently demonstrated a new concept for the simultaneous sampling and analysis of two different epidermal biofluids with a single wearable platform through combined iontophoresis (Fig. 4f). This was accomplished by coupling sweat stimulation (iontophoretic drug delivery) with ISF extraction (via RI), enabling concurrent analysis of biomarkers in each biofluid. The system allows on-demand, controlled simultaneous sampling of two biofluids at physically separated locations on a single wearable tattoo platform. The biosensing performance has been demonstrated by measuring sweat alcohol and ISF glucose as model analytes with human subjects consuming food and alcoholic drink. For continued progress and before real-world use, next-generation non-invasive epidermal biosensing systems would require detailed studies of the correlations to blood levels.
Challenges and future prospects.
The representative examples discussed in this section highlight the tremendous recent progress of epidermal wearable platforms for non-invasive monitoring in sweat or ISF and the great future promise of such wearable epidermal biosensors. Substantial advances have thus been made recently in terms of device integration, sensing accuracy, sweat/ISF generation and replacement, signal transduction, data transmission, and multiplexed sensing, along with related flexible and self-healing materials. Despite these advances, extensive efforts are still required to realize their full diagnostic potential, which should focus on the viability of extended use, critical correlation of sensor response to concurrent analyte blood concentrations, and efficient, controlled sampling of the target biofluids. Further attention is also needed to enhance sweat sampling and transport to improve detection reliability and relevance for monitoring dynamically changing concentrations. Multiplexed sensing platforms can further enhance the reliability of monitoring sweat analytes by correcting for variations in sweat flow, temperature, humidity and pH. The reported systems are particularly applicable in fitness monitoring, which fulfills the requirement of sweat generation through physical exercise. However, alternative sampling routes are required for increasing the impact of epidermal devices for additional applications (e.g., diabetes monitoring or alcohol monitoring). Moreover, non-invasive monitoring of new target biomarkers is desired to broaden the scope and impact of wearable biosensing systems.
Ocular wearable biosensors
Another biological fluid that can be exploited for monitoring physiological status is tears. Not only do biomarker molecules in tears diffuse directly from the blood and exhibit close tear–blood concentration correlations, but also tear analysis presents opportunities for the diagnosis of ocular disease. Tears are also less complex than blood and are part of the antifouling mechanism of the eye. These characteristics make human tears an attractive diagnostic biofluid for healthcare monitoring applications that can be sampled without blood contact94-98.
Secretion and composition of tears.
Human tears, or lachrymal fluid, are secreted by the lachrymal gland as one of the protecting films covering the eye. Tears contain both low and high molecular weight compounds, such as protein/peptides, lipids, metabolites and electrolytes. In particular, tear glucose concentrations have been demonstrated to exhibit good correlation with blood glucose levels, reflecting the diffusion from the lachrymal artery when the tears are sampled without any eye irritation or stimulation, which can compromise the relationship94, 95, 99-101.
Despite the demonstrated correlations, sampling tears for in vitro diagnoses is associated with several errors that are due to the following: first, small sample volumes102; second, ease of evaporation during sample collection; third, variations in tear production among individuals and throughout the day103; and lastly, challenging collection methods that can affect the sampled analyte concentrations104,105. The accuracy of such in vitro tears diagnostic assays thus depends strongly on the collection method, with the most common strategies being via glass capillary tube or the Schirmer’s strip106. Reflex tears, generated during emotional or mechanical stimulation, have different compositions than basal tears, which make up the protective tear film covering the eye surface at all times. These variations and challenges highlight the need for developing wearable tears sensing platforms without eye-irritation.
Tear-based wearable biosensors.
Contact lens-based systems represent an attractive solution to tear collection issues as they can be worn without eye irritation and are in direct constant contact with basal tears94,97,98. These devices integrate all the necessary biosensing, data processing, and power sources within the contact lens platform, which can lead to challenging design requirements. The rapid development of soft materials used for contact lens fabrication offers high degrees of flexibility to minimize eye irritation and avoid discomfort to the wearer. These materials also provide the oxygen permeability necessary to avoid oxygen deficiencies and enhance the accuracy of continuous metabolite monitoring. Contact lens-based sensors were initially introduced utilizing optical detection of tear glucose levels based on the interaction of glucose with concanavalin A or phenylboronic acid derivatives107,108.
The possibility of quantifying glucose in human tear fluid at physiological conditions using holographic contact lenses was also presented around the same time based on its advantages such as a no need of battery, easy reading, and continuous glucose signal monitoring109. Other contact lens-based optical sensors have involved the use of photonic crystal materials in combination with responsive hydrogels or fluorescent dyes for measuring glucose and other target analytes in tears110-113. The combination of such optical sensors with the use of smartphone-based microscopes, including algorithm-based applications, is expected to readily facilitate the readout of the biosensor response.
Further advances for the field came with the demonstration of electrochemical biosensing by the Parviz team114-116. This group investigated different biosensing strategies to achieve good sensitivity, linearity and accuracy. Also, they were able to resolve interference issues by introducing the ‘dual sensor setup’ that implements additional control (GOx-free) working and counter electrodes114,115. Further progress has since been made by embedding a wireless read out chip (2.4 GHz) and by powering with far-field electromagnetic radiation (3μW within 15 cm)116.
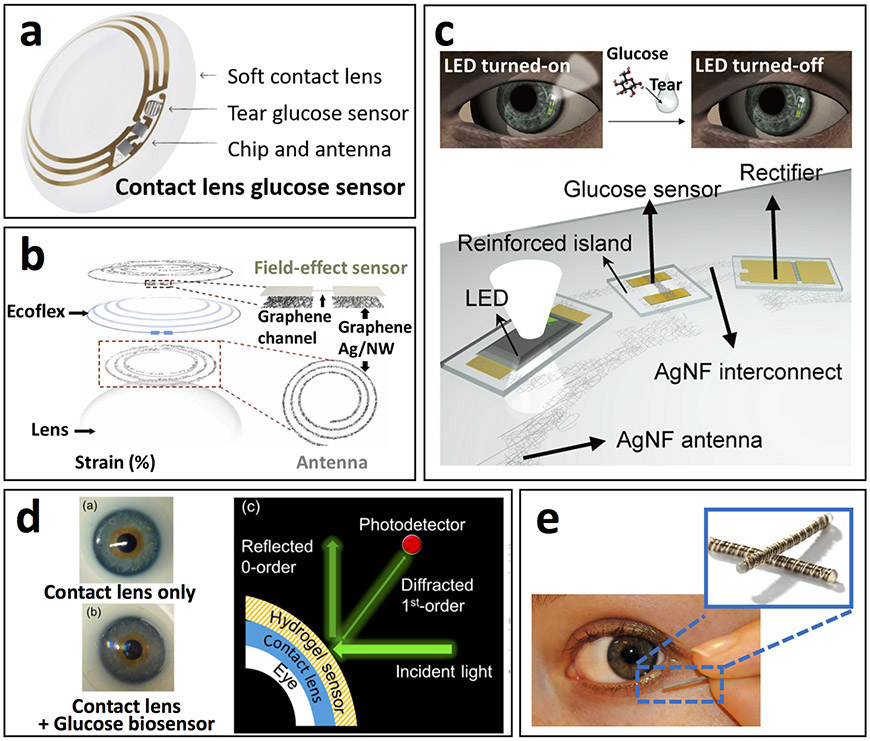
As a result of these consistent endeavors, Google, in partnership with Novartis, made the most noticeable progress by applying their expertise in electronics miniaturization and applied medical technologies, respectively, toward development of a contact lens sensing platform for tears glucose monitoring33. The developed prototype lens consisted of a wireless control chip, miniature electrochemical transducer, and antenna on a soft contact lens platform with the glucose sensor embedded within a hydrogel matrix to non-invasively measure glucose concentrations in the surrounding tears (Fig. 5a). This industry partnership has been projected to accelerate the introduction of contact lens-based biosensors to the commercial market. However, there have been delays to the clinical trials and subsequent commercial release of this product, indicative of the technological challenges of successfully achieving a high-performing contact-lens-based sensing platform.
Figure 5.
Tear-based biosensors. (a) Pictorial depiction of contact lens sensor previously under co-development by Google and Novartis to measure tears glucose concentrations in a miniaturized glucose sensor. Prototype platform contained integrated electronics for sensor response processing and wireless transmission (adapted from https://sites.google.com/site/smartcontactlens/). (b) Schematic illustration of multifunctional wearable smart sensor system incorporated onto contact lenses for monitoring of glucose in tears as well as intraocular pressure using enzyme-functionalized graphene-silver nanowire hybrid nanostructures. The device proved capable of wirelessly detecting fluctuating glucose concentrations and pressure in a rabbit model in vivo and in a bovine eyeball in vitro (adapted from ref. 117). (c) Schematic representation of wireless glucose sensor incorporated into a contact lens platform with wireless power transfer circuitry and display pixels for a fully integrated and transparent platform that does not hinder vision. This device detected fluctuating tear glucose concentrations through a resistance-based enzymatic mechanism, which was demonstrate in a rabbit model (adapted from ref. 118). (d) Pictorial depiction of wearable contact lens tear glucose biosensor platform applied to an artificial eye with schematic representation of smartphone-based quantification of glucose levels through reflection of incident light by the photonic microstructure within the lens. The smart contact lens system integrated with a glucose sensitive hydrogel monitored changing glucose concentrations in vitro without complicated fabrication procedures that allowed rapid response time for continuous measurements (adapted from ref. 112). (e) Depiction of Noviosense electrochemical tear glucose sensor. A small spring-like sensing device designed to be placed within the conjunctive fornix for continuous access to tears glucose (adapted from http://noviosense.com).
Recently, ‘smart’ contact lenses for wireless ocular diagnostics have been further developed by combining glucose and ocular pressure contact lens sensors for wireless in vivo glucose monitoring in the eye of a rabbit, together with in vitro monitoring of ocular pressure using a bovine eyeball (Fig. 5b)117. Although this device is capable of multiplexed sensing, the simultaneous demonstration of two functionalities has not yet been evaluated. Cross-talk and accuracy along with biocompatibility should be critically assessed in further studies with human subjects. The work was further expanded for integration of wireless power transfer circuits and displays on contact lens biosensors, visualizing the in vivo rabbit tear glucose response in real-time (Fig. 5c)118. This advanced device focused on ensuring wearer comfort without hindrance to vision through the use of transparent soft materials, while integrating wireless electronics to eliminate the need for an external power source. However, additional studies are yet required to further demonstrate sensing performance in vivo with human subjects to show viability of practical use in variations of glucose levels throughout a day.
Another recent advance in wearable contact lens biosensors involved the use of smartphones for optical continuous glucose monitoring112. A hydrogel-based sensor with photonic microstructure was attached on top of a commercial contact lens and the reflective power was recorded using a smartphone in response to changes in the tears glucose levels (Fig. 5d). This device offers fast and easy fabrication along with a rapid and sensitive glucose response. Such capabilities indicate an attractive alternative to electrochemistry-based contact lens biosensors, and address challenges with miniaturization in power transfer and data communication.
In addition to contact lens platforms, a small spring-like electrochemical sensor, consisting of multiple coiled wire electrodes, and coated with a protective polysaccharide-based hydrogel material, was designed by Noviosense (http://noviosense.com) for placement in the inferior conjunctival fornix toward constant access to tear fluid (Fig. 5e). Such sensor placement at the base of the eye (behind the eyelid) provides continuously accessible tear glucose measurements when coupled with wireless data transmission, without causing discomfort. A recent clinical trial demonstrated great correlation between tear and blood glucose concentrations in animals and humans (including patients with type 1 diabetes)119.
Challenges and future prospects.
Overall, tear-based sensors have been focused primarily on glucose monitoring but show considerable promise for non-invasive sensing of other physiologically important biomarkers. The scope of new tear analytes can thus be expanded to additional metabolites and key electrolytes that display close relationships with blood. For example, direct tears-based non-invasive assays of tear catecholamines may also be developed for improving the diagnosis of glaucoma120 As tear fluid contains thousands of proteins—the most abundant of which are lysozyme, lactoferrin and albumin—non-invasive tear monitoring could also be used to detect protein biomarkers correlated with disease121. Tear proteome analysis may be one approach for identifying such biomarkers linked with ocular disease. However, as with sweat, these applications require extensive validation of the tear–blood concentration correlation as well as a validation of the importance of a biomarker in ocular disease progression, along with a greater understanding of the tear chemistry in general. A related challenge is to better understand the influence of the sampling procedure upon tear composition.
Wearable contact lens tear monitoring platforms are highly advantageous as they do not cause any eye irritation and yield a relatively consistent tear fluid composition. Such systems have already proven attractive for monitoring health status and can be further expanded toward therapeutic applications with potential capability enhancement by miniaturization of the electronic interface and power source toward full integration onto the lens. Microfluidics could also be applied for addressing challenges of tears sampling, such as small volume and ease of evaporation toward real-time accurate tear monitoring. Such a fluidic platform was suggested by the Butt group112 for integration with an optical monitoring system, but has not been demonstrated for biosensing applications. The successful realization of this idea would greatly enhance the accuracy of future tears biomonitoring.
Because of the sensitive nature of the eye to foreign objects, in vivo evaluations of tear biosensors currently rely on animal studies, but further efforts and safety measures should lead to practical applications with human subjects. Compared with epidermally focused wearable biosensors, tear-based systems benefit from the advantage of continuous access to the target biofluid without the need for induction or extraction. However, difficult sampling procedures complicate reliable tear-based sensing platforms while contact lens-type systems suffer from design constraints imposed by the nature of their operating environment.
Oral-cavity wearable biosensors
The interest in saliva as a diagnostic fluid has advanced rapidly in recent years122. Many of the biomarkers in saliva pass directly from the bloodstream via transcellular or paracellular paths, making saliva the “mirror of the human body” that reflects the body’s physiological state to offer a non-invasive alternative to blood analysis. The high protein content of saliva makes it attractive for detecting disease and stress biomarkers toward biomedical and fitness monitoring. Because saliva can be readily collected123-125, it has been used in connection to in vitro diagnostic biosensors on strips or portable device platforms,126-134.
Secretion and composition of saliva.
Saliva is a complex oral fluid, which is produced mainly by the parotid gland and is composed of numerous constituents, such as metabolites, enzymes, hormones, proteins, microorganisms and ions125,135-140. Several of these saliva biomarkers (e.g., drug, hormones, metabolites or antibodies) have been used in clinical settings as they offer meaningful diagnostic information140-144. However, few studies have focused on developing wearable oral cavity biosensors, likely due to potential biofouling effects caused by the rich salivary protein content and the very low concentration of some target biomarkers. Despite these challenges, in-mouth biosensing platforms can offer an attractive painless route for obtaining dynamic chemical information from saliva. Oral wearable platforms require the incorporation of biosensor and electronic interface into an orally mounted device, such as a mouthguard or denture-based system.
Saliva-based wearable biosensors.
The first example of a wearable oral sensor was demonstrated in the 1960s and was based on a partial denture platform for monitoring mastication monitoring plaque pH and fluoride concentrations. However, such in-mouth operation required the replacement of several teeth by the sensors and was subject to risks associated with potential leakage of the internal sensor solution. The field of oral biosensing was expanded by Mannoor et al.32, who reported graphene-based nanosensors printed onto water-soluble silk and transferred directly onto tooth enamel for passive, wireless bacteria detection. These oral-cavity sensors were integrated with a resonant coil for battery-free operation and were capable of detecting salivary bacteria at the single cell level in vitro using a naturally occurring antimicrobial peptide (AMP) biorecognition element along with label-free impedance transduction. This attractive wearable biosensing concept was targeted at remote monitoring of bacterial film development on the teeth and could be expanded to the extended monitoring of other salivary biomarkers.
The early demonstrations of oral-cavity sensor capabilities and potential, coupled with multiple in vitro studies that showed good correlations between blood and salivary metabolite levels, encouraged recent research efforts toward the development of modern oral cavity salivary metabolite sensors, particularly in connection to wearable mouthguard platforms. Our group (J.K., J.W. et al.)145 was the first to develop mouthguard-based salivary metabolite electrochemical biosensors through integration of screen-printed enzymatic electrodes. Salivary lactate has shown good correlation with blood lactate levels, toward assessing physical stress and performance146,147. This device is capable of detecting salivary lactate electrochemically in connection to selectivity imparted by the lactate oxidase enzyme; protection against biofouling in undiluted human salivary samples was conferred by electro-polymerized ortho-phenylenediamine (OPD) for continuous, non-invasive physiological monitoring of an individual’s fitness state.
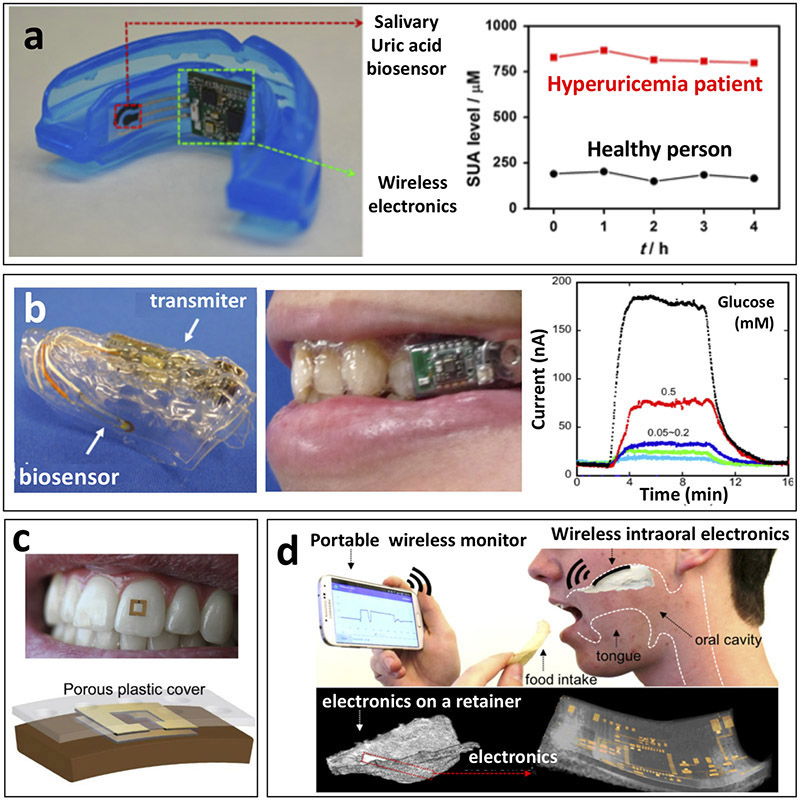
We (J.K., J.W. et al.)36 have further continued development of oral-cavity sensors by demonstrating a mouthguard-based uric acid biosensor that incorporated anatomically miniaturized instrumentation electronics featuring a potentiostat, microcontroller, and a Bluetooth low energy (BLE) transceiver for monitoring salivary uric acid levels toward clinical applications (Fig. 6a). This platform enables non-invasive monitoring of blood uric acid levels, which is a biomarker for various diseases (e.g., hyperuricemia, gout and renal syndrome), and it displays highly sensitive, selective, stable and rapid response toward obtaining dynamic chemical data on salivary biomarkers in the oral cavity. Although these mouthguard-based biosensing devices are well suited for fitness or diagnostic applications, more discrete platforms would be required for extended use, such as for continuous glucose monitoring in daily life.
Figure 6.
Saliva-based biosensors. (a) Depiction of mouthguard-based wearable salivary uric acid biosensing platform with integrated wireless electronics and analysis of salivary uric acid concentrations in a healthy volunteer and a hyperuricemia patient. This platform exhibited selective uric acid detection in undiluted human saliva to monitor the response or uric acid levels of a hyperuricemia patient during treatment (adapted from J.K., J.W. et al.36). (b) Depiction of mouthguard-based sensor for glucose monitoring in saliva with on-body application and analysis of increasing glucose concentrations. Fully integrated saliva glucose sensor toward continuous in-mouth glucose monitoring (adapted from refs 97,148). (c) On-body depiction and cross-sectional configuration of radiofrequency trilayer tooth-mounted sensor for wireless monitoring of food consumption. This dielectric sensor fabricated with biocompatible materials was capable of being mounted onto tooth enamel to detect foods and fluids during ingestion when functionalized with analyte sensitive layers. Projected uses were for detection of sugars, alcohol, salinity, pH and temperature (adapted from ref. 155). (d) Depiction of operational principles and electronics configuration of wireless, user-comfortable sensing platform for long-range oral monitoring of sodium intake toward hypertension management. Electrochemical sodium sensing was demonstrated in vitro as well as in vivo with the orally-mounted biocompatible sensing platform (adapted from ref. 156).
Oral biosensing devices have been further miniaturized to a detachable ‘cavitas sensors’ device to measure salivary glucose levels on a mouthguard platform fabricated to fit over the wearer’s teeth (Fig. 6b)148. The developed sensors are based on a GOX-modified poly(ethylene terephthalate) glycol (PETG) surface, and the device is seamlessly integrated with a wireless transmitter on a custom-fitted monolithic mouthguard platform. This configuration enables the telemetric measurement of salivary glucose in artificial saliva over the relevant physiological range (5–1000 μM) and the device has been further characterized through connection to a phantom jaw that mimics the human oral cavity with a saliva flow system.
As demonstrated in the above studies, salivary glucose has been highlighted as having a positive correlation with blood glucose levels. This correlation reflects the diffusion and active transport of blood components to the salivary gland144. The direct relationship between blood glucose and salivary glucose provides a highly advantageous, easily accessible route for glucose sampling. In the case of diabetes, changes in hormonal and neural balance may impact the salivary glands, which act as a filter from the blood, and lead to increased secretion of salivary glucose. Salivary glucose can thus offer an alternative painless screening route for diabetic patients149-154. Soni et al.126 have demonstrated the correlation between blood and saliva glucose concentrations to be R = 0.64 in healthy subjects, whereas diabetic subjects show a much closer relationship with R = 0.95. However, further large-population studies are required before considering the use of salivary glucose for screening or monitoring diabetes in integration with a wearable miniaturized platform.
Another wearable sensor based on an oral-cavity platform recently has been demonstrated using in-mouth operation with a human subject (Fig. 6c)155. Such oral sensing was realized by introducing biocompatible materials, such as porous silk and hydrogels, on a tooth-mounted oral cavity sensor capable of wireless monitoring of foods during ingestion. In vivo use was demonstrated through the measurement of different fluid properties, such as alcohol content, salinity, sugars, pH and temperature using RF sensors. However, bringing this strategy to practical real-life applications would require critical evaluation of the selectivity toward the target analytes for ensuring accurate data.
An alternative in vivo oral monitoring device has also been developed for sodium intake via long-range wireless telemetry (Fig. 6d)156. This oral sensing platform relies on a user-comfortable system using ultrathin stretchable electronics along with miniaturized sensors. The performance of the device has been demonstrated with human subjects, proving its feasibility for real-time monitoring of sodium consumption, which is desired for managing hypertension. However, the toxicity of the device was evaluated without the chemical sensing layer, and hence practical oral-cavity applications would require further critical assessment along with a biocompatible recognition layer. Further efforts are also needed for measuring sodium uptake during food/drink consumption. Overall, the recently developed in-mouth sensing platforms require additional critical evaluations to ensure the safety and reliability necessary for future deployment of such oral-cavity systems. Particular attention should be given to minimizing surface fouling and contamination effects caused by other saliva constituents and food debris, respectively, and for ensuring the safety of these devices.
Challenges and future prospects.
Despite the promise of saliva as a non-invasive diagnostic fluid, challenges remain regarding the realization of widespread accurate oral monitoring applications. The concentrations of many important biomarkers in saliva are substantially lower than in blood, requiring highly sensitive sensing systems for accurate monitoring. Compared with other non-invasively sampled biofluids, saliva can be readily sampled without complicated procedures, but is composed of a rich matrix of constituents that can also be easily contaminated by external factors (e.g., food and drink). Caution must also be used to avoid potential gum bleeding, which would lead to contamination or false signals. The high concentrations of protein in saliva, including mucins and proteolytic enzymes, along with food debris, can lead to rapid biofouling of the oral cavity sensor through non-specific absorption at the transducer surface. Such challenges can be addressed by developing permselective protective sensor coatings that exclude macromolecules from the surface.
Future work toward practical in-mouth applications requires detailed validation studies in comparison with blood, and critical assessment of safety issues, such as biocompatibility, potential toxicity, sterilization, and operational stability for in-mouth operation. Effective device encapsulation (including the supporting electronic interface and power supply) and use of biocompatible materials are essential for eliminating risks related to their contact with the saliva fluid (in particular, chemical leaching to the surrounding fluid). Such encapsulation is also essential for protecting the functionality of the electronics. Continuous discovery of new saliva biomarkers will further be helpful for expanding the diagnostic scope of saliva. Such diagnostic capabilities can benefit from the introduction of multiplexed oral cavity biosensors.
Prospects and challenges
In this review, we have highlighted the most prominent approaches and the latest progress involving representative examples of modern wearable biosensors. A plethora of innovative wearable biosensing devices have already been demonstrated in diverse applications, ranging from the detection of metabolites (e.g., lactate or glucose) to the monitoring of electrolytes (e.g., sodium, potassium or calcium) in fluids, such as sweat, ISF, saliva or tears in connection to enzymatic and ion-recognition reactions. These demonstrations have shown that wearable biosensors have immense potential for real-world applications. Such dramatic progress has benefited from the refinement of multiplexed-sensing platforms, improved biofluid sampling and advances in flexible materials and wireless electronics. These advances have greatly enhanced the reliability of wearable biosensor, the analyte monitoring capabilities and wearability.
Despite tremendous recent progress in wearable biosensors, the state-of-the-art in this field remains at demonstrating proof-of-concept wearable biosensing platforms for detecting several representative biomarkers, and only small steps can be taken to practical applications in the field. Wearable biosensors face many fundamental challenges and technological gaps related to the scope, validation, stability and accuracy, along with power (Box 1)1,42,157-167, communication (Box 2)1,168 and security/privacy issues (Box 3)1,169-172 Overcoming such technological challenges is critical to the successful growth of wearable biosensors toward widespread commercial realization. Some of these challenges are specific to the individual platforms or target analytes, whereas others are shared by all wearable biosensing systems.
Box 1. Wearable biosensor power challenges1,42,157-167.
The majority of wearable biosensor power consumption arises from three main sources: first, powering of the sensors detecting the biomarkers (with a concomitant increase in power consumption as sensing is increasingly multiplexed); second, data processing, which is a trade-off between energy consumption and data collection rate; and finally, wireless communication (both communication with other integrated sensors and data transmission). Meeting these power needs successfully requires multiple approaches180-190. These include safe high-energy (wearable) batteries, alternative energy harvesting/storage devices (e.g., biofuel cell, solar cell, thermoelectric, piezoelectric/triboelectric, supercapacitor, or a combination of sources), energy efficient sensing devices, and self-powered biosensors based on biofuel cells utilizing the analyte as the fuel. Furthermore, controlling and adjusting the sampling frequency and data transmission - based on the desired information and wearer activity - could allow efficient power consumption. For example, storing the vital information during ‘sleep time’ without communicating to the end user greatly reduces power consumption from data transmission.
Box 2. Wearable biosensor communication challenges1,168.
To realize their full potential, wearable biosensors must be capable of wireless communication among individual wearers, multiplexed biosensors, and computing devices. They must also be capable of long-distance data transmission, and achieve these tasks in an energy efficient manner.
When assessing a communication protocol for a device, several criteria are taken into account: device power consumption; amount of data generated; required bandwidth; and compatibility with sensor circuitry. Strategies for wireless communication include the following (J.K. & J.W.)191: first, Bluetooth—or most commonly BLE (Bluetooth low energy)—, which consumes less power, but also has the limitation of a 100 m distance limit for reception; and second, NFC (near field communication)/RFID (radio frequency identification), which is battery-free, but requires close proximity for signal reception.
Currently, periodic data transmission depending on the desired biochemical information and energy requirements is one approach being explored to address the communication constraints of current devices. Another is the development of radical wireless technologies for long-distance data transmission within a high-density network of wearable devices.
Box 3: Data security in wearable biosensors1,169-172.
A key benefit of utilizing wearable biosensors for healthcare applications is the ability to monitor patients’ health status in real-time, remotely and continuously. Yet, these advanced capabilities could cause users harm if the hardware and software systems are not designed with security and privacy in mind. Access to individual’s biomedical data should be strictly limited to authorized users, without compromising privacy. Further, collected biomarker metrics that lead to actionable interventions (such as drug delivery) could have life threatening or serious health consequences. While comprehensive protection of collected data is critical for a reliable safe operation, such data protection is challenging considering the goal of seamless connectivity and wireless data transmission. The network connected to a user’s sensor data has a significant potential risk of outsider and insider security attacks. The threat of an outsider’s attack can be mitigated by reliable authentication/encryption techniques. Data security risks become more severe in cases of an insider’s attack, with those possessing legitimate access to raw data. For example, the attacker can add false health data that may impact on patient’s diagnosis and treatment. Early detection of such intrusion will minimize security risks and will ensure the confidentiality, dependability, and integrity of healthcare data generated by wearable biosensors.
Quite apart from these security issues, the high data volume arising from continuous real-time measurements using wearable biosensors requires the implementation of intelligent data processing protocols (J.W. et al.)1. Some approaches that are commonly applied to data derived from wearables include data cleaning and filtering processes (e.g., noise filtration) that can reduce wireless data transfer rates and energy consumption. Furthermore, expanded data mining protocols can predict unforeseen situations and establish correlations between sensor signal and clinical diagnostics among a large population of wearers.
Toward measurement of a wider range of biomarkers.
Most current wearable biosensing devices measure a small number of biomarkers. Going forward, greater efforts should focus on new biosensor formats and improved knowledge of non-invasively sampled biofluids to monitor a wider range of biomarkers. Understanding the composition of each biofluid and its relation to blood chemistry and to certain medical disorders will be essential for expanding the reach of wearable technology in the healthcare arena, and for the widespread acceptance of these devices by the clinical community. The real-time correlation of analyte levels in non-invasive biofluids to concurrent blood concentrations is crucial to such acceptance. Rigorous and reproducible interpretation of biosensor readings in the real world is also an ongoing goal, particularly in applications where clinical or actionable responses may be required.
Going forward, a systematic, in-depth analysis of the composition of each of the different biofluids will be vital for identifying new biomarkers (e.g., metabolites, proteins and nucleic acids) that previously have remained out of the scope of wearable sensors. Moreover, the evaluation of their dynamic concentration fluctuations under different scenarios may also provide new insights into circadian rhythms, disease trajectories and wellness over time on-invasive sensing may also be expanded beyond the measurement of a few limited metabolites and electrolytes as currently practiced to a situation in which a whole slew of protein disease markers, hormones and stress markers are assessed using non-invasive immunoassays. Similarly, further opportunities may open up in exploring new types of bodily fluid (beyond ISF, sweat, tears and saliva), such as urine, mucus and semen. Such real-time analysis of a wider range of biomarkers in a wider range of biofluids would ultimately benefit other areas of biomedicine, such as biomarker-directed clinical development of new experimental therapies.
New wearable immunosensors will require advanced microfluidic platforms, carrying multiple steps (e.g., tagging, washing and receptor regeneration common to bioaffinity assays), along with long reaction times for detecting very low biomarker concentrations. Such on-body bioaffinity assays could be simplified using label-free detection schemes173,174. Future wearable immunosensors hold considerable promise not only for healthcare and fitness applications but also for a variety of biodefense applications.
The integration of multi-analyte sensing will be essential to the future adoption of wearable biosensors for tracking wearer health state at the molecular level. Although most early wearable devices have focused primarily on single measurements, efforts should continue toward the simultaneous, non-invasive monitoring of a wide panel of biomarkers. This more comprehensive analysis can give not only a broader analysis of the physiological state, but also provide for active calibration and correction of the response for more accurate monitoring. Furthermore, incorporation of multiple sensing approaches for the same analyte could lead to improved biosensor reliability. Efforts should also continue to emphasize the development of multi-modal wearable sensors that fuse chemical, electrophysiological and physical sensors. The combination of different wearable sensor modalities should lead to a more comprehensive monitoring of human physiology and could find widespread applications, ranging from monitoring neonates to the monitoring of the elderly.
Although access and real-time analysis of non-invasively sampled biofluids promises new and rich diagnostics information, successful realization of wearable biosensors in healthcare will require extensive validation and large-scale correlation studies with gold-standard blood-based clinical assays. These rigorous large population studies—and the correlation of wearable sensor data with data from blood assays—will be requisite for developing reliable and safe wearable biosensing diagnostic platforms and will likely necessitate further connection to large-scale medical data mining, the Internet of Things (IoT), cloud computing and machine learning methods to take full advantage of the data in the context of healthcare settings.
Accuracy and stability.
Ensuring that wearable sensor responses are both accurate and reliable will be critical to their acceptance in the marketplace. Accuracy is often compromised by surface-fouling effects, which represent a major challenge to the continuous operation of on-body biosensors. During such applications, repetitive measurements are carried out over an extended period of time. To ensure the reliability of the response during prolonged on-body operation, robust anti-fouling surface protection is desired along with active calibration mechanisms (e.g., multi-modal, multi-analyte sensing and drift correction). Biofouling is driven by the accumulation of proteins, cells or macromolecules on the sensor surface through non-specific binding. Such rapid adsorption impedes the diffusion of the target analyte to the sensor surface, leading to a gradual decrease of the sensing signal over the time. Fouling of the surface of wearable biosensors, particularly those operating in sweat or tears over short durations, is expected to be less severe compared with that observed with implantable or minimally-invasive sensors. In contrast, substantial biofouling is expected for saliva-based oral-cavity biosensors as the complex saliva matrix contains a much higher protein content compared to other non-invasive biofluids (e.g., sweat or tears). Such oral-cavity biosensors would thus require special attention to the surface protecting coatings. The sensor coating materials should be carefully selected for minimizing biofouling effects and excluding co-existing electroactive interferences, while retaining enzyme at the sensor surface and avoiding leakage of potentially toxic sensor components.
Unlike traditional (lab-based) biosensors, wearable biosensors can be exposed to harsh and fluctuating conditions (e.g., temperature) during prolonged outdoor activities in uncontrolled environments. Such severe conditions may affect the stability of their fragile bioreceptors. Multiplexed sensing, including both biosensors and physical sensors, could provide active calibration for variations in temperature, pH and humidity.
Achieving wearable biosensors with long-term operational and storage stabilities requires a proper attention to the receptor immobilization, surface chemistry, and storage conditions. Accurate on-body measurements also require attention to potential contamination from the surroundings, carry over from mixing with old fluid, and continuous signal drift (and related sensor calibration). These issues can be partially addressed using proper microfluidic sampling systems and optimization of surface coating techniques.
System integration and hardware.
In addition to challenges stemming from biosensing components, the integration of effective hardware is essential for the overall device operation and for the successful realization of these wearable platforms. Although not limited to wearable biosensors, attention to hardware, power and communication issues is crucial for the practical utility of these sensing devices. The hardware components must have a high level of integration with the biosensor platform and have varied requirements depending on the specific application. A wireless electronic printed circuit board (PCB) containing a fully functional microcontroller is a widely used platform for wireless electronics due to its flexibility and cost-effectiveness. The PCB can be further integrated with a battery (the most commonly used power source) by encapsulation in biocompatible insulating materials (e.g., parylene-C) for safety. Another key requirement for wearable devices is maintaining low power consumption during continuous prolonged monitoring while providing the wearer (and other end users) with useful timely chemical information. This may require a trade-off between energy consumption and data rate, particularly when high sampling frequency is required. Efficient data processing and effective and safe communication of the collected data are also extremely important requirements.
New data mining algorithms are being developed for making sense of the large amount of data and predicting patterns in connection to specific applications. The careful interpretation of collected data and thorough verification of the biosensor response is vital for wearable devices which monitor conditions used for follow-up clinical action, by the wearer or autonomously via a closed-loop system (e.g., glucose monitoring leading to insulin delivery).
The provision of power to wearable biosensing platforms (see Box 1) can be accomplished in several ways, with the most common approach being lithium ion or alkaline batteries. These devices exhibit long lifetimes for extended biosensor operation; however, they are relatively bulky and can pose toxicity issues, particularly lithium ion-based systems175,176. Alternatively, wearable batteries have been developed on stretchable and flexible materials for greater wearability, but have not yet demonstrated sufficient energy density for long-term use177-179.
Major advances have been made in wearable supercapacitor technology as well. These materials have shown fast charge and discharge capacity, but also possess low gravimetric and volumetric energy densities180-182. Wearable power sources have also been reported that harvest energy during operation, depending on the type of wearable platform being powered. Energy can be harvested from light with wearable solar cells183,184, motion with piezoelectric or triboelectric devices185,186, heat with thermoelectric materials187,188, or chemical constituents of the sampled biofluids with wearable biofuel cells189-192. Wearable biofuel cells, in particular, are promising for the powering of non-invasive wearable platforms as they harvest energy from the same biofluids of interest and can operate as self-powered biosensors, but yet still suffer from the same stability issues discussed above42,193,194.
One way of maximizing benefits is to combine several of the above approaches. For instance, a wearable supercapacitor might be implemented to store energy harvested using a wearable biofuel cell when the energy is not immediately needed. Advances in wearable power sources are a significant need, particularly with increases in energy demands caused by multiplexed sensing platforms. Researchers have focused on a combination approaches that involve the development of both the power sources and the design of more energy-efficient devices and adaptive algorithms to decrease energy demands. In-depth discussion of such research has been extensively covered elsewhere (J.W. et al.)1.
Translation to the commercial market.
The global wearable sensors market was valued at roughly $150 million in 2016 and is projected to reach $2.86 billion by 2025, according to a recent market report (https://www.grandviewresearch.com/industry-analysis/global-wearable-sensor-market). A large portion of this future market share is expected to be made up of wearable biosensors, primarily non-invasive glucose monitoring2. The slower than anticipated rate of introduction of non-invasive biosensing platform to the commercial market has depressed market expectation; however, with the rapid growth of research reports and proof-of-concept demonstrations, many are still bullish about the market prospects of wearable biosensors.
The successful translation of wearable biosensors and proof-of-concept demonstrations to the commercial market faces several hurdles related to their fundamental operation. For completely reliable use, wearable devices must overcome stability issues caused by prolonged operation under uncontrolled conditions, biofouling from constituents present in the sampled biofluids, and intrinsic instabilities of the biological recognition components themselves. Furthermore, the devices must be capable of robust operation without the need for constant (re)calibration (common in laboratory settings). The sensor preparation thus must ensure high bioreceptor stability for maintaining accuracy and reliability of the response. Also, a proper fluid sampling system, such as microfluidics, is desirable to provide effective and rapid transport of the biofluid over the sensor and ensure a reproducible and accurate signal along with negligible sample contamination and carry over. Such advanced wearable fluidic systems could also facilitate multi-step bioaffinity assays, particularly for immunoassays.
The regeneration issue of immunosensors is another major challenge to overcome if prolonged use of wearables is to be attained. Fully integrated wearable biosensing platforms would require incorporation of wireless electronics (integrated with energy source) to facilitate the data processing and secured signal transmission. Furthermore, the use of mobile devices and smartphone-based microscopes, including algorithm-based applications, is expected to facilitate the readout of the response in optical wearable biosensors112,113,195. Considering all the above challenges, we are only at the beginning of understanding how wearable biosensor technologies can improve our health and performance.
What’s next?
Future wearable biosensors are expected to become more streamlined, moving away from the wrist into textiles and fashion accessories that blend further into a wearer’s daily life. Some of these devices will require disposable components to address fouling issues. Such future wearable biosensors will monitor non-invasively a wide range of biomarkers (including proteins and nucleic acids), ultimately enabling a comprehensive medical diagnostics and performance assessment. The acceptance of these non-invasive biosensors by the medical community will require extensive and successful validation in human testing and improved understanding of the clinical relevancy of sensor information. Given the competitive research and tremendous commercial opportunities in wearable biosensors, we anticipate exciting new developments in the near future. The wearable sensors market is thus expected to continue its rapid growth and continue its trajectory to changing and improving people lives. Such future advances, breakthroughs and growth will require close multidisciplinary collaboration among the engineering, scientific and medical communities.
Acknowledgments
This work was supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense (HDTRA 1-16-1-0013). A.S.C. acknowledges funding through the NIH NIAAA T32 Training Grant AA013525.
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Bandodkar AJ, Jeerapan I & Wang J Wearable chemical sensors: Present challenges and future prospects. ACS Sens. 1, 464–482 (2016). [Google Scholar]
- 2.Heikenfeld J et al. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 18, 217–248 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matzeu G, Florea L & Diamond D Advances in wearable chemical sensor design for monitoring biological fluids. Sens. Actuators B 211, 403–418 (2015). [Google Scholar]
- 4.Liu Y, Pharr M & Salvatore GA Lab-on-skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 11, 9614–9635 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Amjadi M, Kyung KU, Park I & Sitti M Stretchable, skin-mountable, and wearable strain sensors and their potential applications: A review. Adv. Funct. Mater 26, 1678–1698 (2016). [Google Scholar]
- 6.Bariya M, Nyein HYY & Javey A Wearable sweat sensors. Nat. Electron 1, 160–171 (2018). [Google Scholar]
- 7.Clark LC & Lyons C Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N.Y. Acad. Sci 102, 29–45 (1962). [DOI] [PubMed] [Google Scholar]
- 8.Abdellatif MS, Suleiman AA & Guilbault GG Enzyme-based fiber optic sensor for glucose determination. Anal. Lett 21, 943–951 (1988). [Google Scholar]
- 9.Arnold MA Enzyme-based fiber optic sensor. Anal. Chem 57, 565–566 (1985). [DOI] [PubMed] [Google Scholar]
- 10.Seitz WR Chemical sensors based on fiber optics. Anal. Chem 56, 16A–34A (1984). [Google Scholar]
- 11.Ward MD & Buttry DA In situ interfacial mass detection with piezoelectric transducers. Science 249, 1000–1007 (1990). [DOI] [PubMed] [Google Scholar]
- 12.Muramatsu H, Dicks JM, Tamiya E & Karube I Piezoelectric crystal biosensor modified with protein-A for determination of immunoglobulins. Anal. Chem 59, 2760–2763 (1987). [DOI] [PubMed] [Google Scholar]
- 13.Janshoff A, Galla HJ & Steinem C Piezoelectric mass-sensing devices as biosensors - an alternative to optical biosensors? Angew. Chem. Int. Ed 39, 4004–4032 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Ngeh-Ngwainbi J, Suleiman AA & Guilbault GG Piezoelectric crystal biosensors. Biosens. Bioelectron 5, 13–26 (1990). [DOI] [PubMed] [Google Scholar]
- 15.Hilditch PI & Green MJ Disposable electrochemical biosensors. Analyst 116, 1217–1220 (1991). [DOI] [PubMed] [Google Scholar]
- 16.Frew JE & Hill HAO Electrochemical biosensors. Anal. Chem 59, 933A–944A (1987). [DOI] [PubMed] [Google Scholar]
- 17.Bindra DS et al. Design and in vitro studies of a needle-type glucose sensor for subcutaneous monitoring. Anal. Chem 63, 1692–1696 (1991). [DOI] [PubMed] [Google Scholar]
- 18.Wilson GS & Gifford R Biosensors for real-time in vivo measurements. Biosens. Bioelectron 20, 2388–2403 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Rodbard D Continuous glucose monitoring: A review of successes, challenges, and opportunities. Diabetes Technol. Ther 18, 3–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng GF, Patolsky F, Cui Y, Wang WU & Lieber CM Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol 23, 1294–1301 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Pokorski JK, Nam JM, Vega RA, Mirkin CA & Appella DH Cyclopentane-modified PNA improves the sensitivity of nanoparticle-based scanometric DNA detection. Chem. Commun,0, 2101–2103 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Thorp HH Cutting out the middleman: DNA biosensors based on electrochemical oxidation. Trends Biotechnol. 16, 117–121 (1998). [Google Scholar]
- 23.Wang J From DNA biosensors to gene chips. Nucleic Acids Res. 28, 3011–3016 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odenthal KJ & Gooding JJ An introduction to electrochemical DNA biosensors. Analyst 132, 603–610 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Wang J Electrochemical glucose biosensors. Chem. Rev 108, 814–825 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Johnson BN & Mutharasan R Biosensing using dynamic-mode cantilever sensors: A review. Biosens. Bioelectron 32, 1–18 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Newman JD & Turner AP Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron 20, 2435–2453 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Mani V, Chikkaveeraiah BV, Patel V, Gutkind JS & Rusling JF Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticle film electrodes and multienzyme-particle amplification. ACS Nano 3, 585–594 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Campbell AS & Wang J Wearable non-invasive epidermal glucose sensors: A review. Talanta 177, 163–170 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Tierney MJ et al. Clinical evaluation of the glucowatch(R) biographer: A continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron 16, 621–629 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Vashist SK Continuous glucose monitoring systems: A review. Diagnostics 3, 385–412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannoor MS et al. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun 3:763 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Senior M Novartis signs up for Google smart lens. Nat. Biotech 32, 856 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Koh A et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med 8, 366ra165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao W et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J et al. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron 74, 1061–1068 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandodkar AJ et al. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem 87, 394–398 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Martín A et al. Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. ACS Sens. 2, 1860–1868 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Sempionatto JR et al. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 17, 1834–1842 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol 11, 566–571 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Emaminejad S et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. P. Natl. Acad. Sci. U.S.A 114, 4625–4630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeerapan I, Sempionatto JR, Pavinatto A, You JM & Wang J Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A 4, 18342–18353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munje RD, Muthukumar S, Jagannath B & Prasad S A new paradigm in sweat based wearable diagnostics biosensors using room temperature ionic liquids (RTILs). Sci. Rep 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imani S et al. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun 7, 11650 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J, Kumar R, Bandodkar AJ & Wang J Advanced materials for printed wearable electrochemical devices: A review. Adv. Electron. Mater 3, 1600260 (2017). [Google Scholar]
- 46.Bandodkar AJ, Mohan V, Lopez CS, Ramirez J & Wang J Self-healing inks for autonomous repair of printable electrochemical devices. Adv. Electron. Mater 1, 1500289 (2015). [Google Scholar]
- 47.Wu H, Gao W & Yin ZP Materials, devices and systems of soft bioelectronics for precision therapy. Adv. Healthcare Mater 6, 1700017 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Stoppa M & Chiolerio A Wearable electronics and smart textiles: A critical review. Sensors-Basel 14, 11957–11992 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bandodkar AJ & Wang J Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 32, 363–371 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Bandodkar AJ, Jia WZ & Wang J Tattoo-based wearable electrochemical devices: A review. Electroanalalysis 27, 562–572 (2015). [Google Scholar]
- 51.Jin H, Abu-Raya YS & Haick H Advanced materials for health monitoring with skin-based wearable devices. Adv. Healthcare Mater 6, 1700024 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Piwek L, Ellis DA, Andrews S & Joinson A The rise of consumer health wearables: Promises and barriers. PLOS Med. 13, 1001953 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y & Gao W Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev (2018). [DOI] [PubMed] [Google Scholar]
- 54.Xue XY et al. Self-powered electronic-skin for detecting glucose level in body fluid basing on piezo-enzymatic-reaction coupling process. Nano Energy 26, 148–156 (2016). [Google Scholar]
- 55.Han WX et al. A self-powered wearable noninvasive electronic-skin for perspiration analysis based on piezo-biosensing unit matrix of enzyme/ZnO nanoarrays. ACS Appl. Mater. Interfaces 9, 29526–29537 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Morris D et al. Bio-sensing textile based patch with integrated optical detection system for sweat monitoring. Sens. Actuators B 139, 231–236 (2009). [Google Scholar]
- 57.Windmiller JR & Wang J Wearable electrochemical sensors and biosensors: A review. Electroanalalysis 25, 29–46 (2013). [Google Scholar]
- 58.Jia WZ et al. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem 85, 6553–6560 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Gualandi I et al. Textile organic electrochemical transistors as a platform for wearable biosensors. Sci. Rep 6, 33637 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caldara M, Colleoni C, Guido E, Re V & Rosace G Optical monitoring of sweat pH by a textile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sens. Actuators B 222, 213–220 (2016). [Google Scholar]
- 61.Choi DH, Kim JS, Cutting GR & Searson PC Wearable potentiometric chloride sweat sensor: The critical role of the salt bridge. Anal. Chem 88, 12241–12247 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Liu R & Xu KX Accuracy of noninvasive glucose sensing based on near-infrared spectroscopy. Appl. Spectrosc 69, 1313–1318 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Ben Mohammadi L et al. In vivo evaluation of a chip based near infrared sensor for continuous glucose monitoring. Biosens. Bioelectron 53, 99–104 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Sonner Z et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thennadil SN et al. Comparison of glucose concentration in interstitial fluid, and capillary and venous blood during rapid changes in blood glucose levels. Diabetes Technol. Ther 3, 357–365 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Campbell AS, Kim J & Wang J Wearable electrochemical alcohol biosensors. Curr. Op. Electrochem 10, 126–135 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fogh-Andersen N, Altura BM, Altura BT & Siggaardandersen O Composition of interstitial fluid. Clin. Chem 41, 1522–1525 (1995). [PubMed] [Google Scholar]
- 68.Venugopal M et al. Clinical evaluation of a novel interstitial fluid sensor system for remote continuous alcohol monitoring. IEEE Sens. J 8, 71–80 (2008). [Google Scholar]
- 69.Venugopal M, Arya SK, Chornokur G & Bhansali S A realtime and continuous assessment of cortisol in ISF using electrochemical impedance spectroscopy. Sens. Actuators A 172, 154–160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bandodkar AJ et al. Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring. Analyst 138, 123–128 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Bandodkar AJ et al. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron 54, 603–609 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Guinovart T, Bandodkar AJ, Windmiller JR, Andrade FJ & Wang J A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 138, 7031–7038 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Kim J et al. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun 51, 41–45 (2015). [Google Scholar]
- 74.Kim J et al. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sens. 1, 1011–1019 (2016). [Google Scholar]
- 75.Wang L et al. Weaving sensing fibers into electrochemical fabric for real-time health monitoring. Adv. Funct. Mater 28, 1804456 (2018). [Google Scholar]
- 76.Moyer J, Wilson D, Finkelshtein I, Wong B & Potts R Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther 14, 398–402 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Sakaguchi K et al. Evaluation of a minimally invasive system for measuring glucose area under the curve during oral glucose tolerance tests: usefulness of sweat monitoring for precise measurement. Journal of diabetes science and technology 7, 678–688 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee H et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv 3, e1601314 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Curto VF et al. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sens. Actuators B 171, 1327–1334 (2012). [Google Scholar]
- 80.Mu X et al. A paper-based skin patch for the diagnostic screening of cystic fibrosis. Chem. Commun 51, 6365–6368 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Huang X et al. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small 10, 3083–3090 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Oncescu V, O'Dell D & Erickson D Smartphone based health accessory for colorimetric detection of biomarkers in sweat and saliva. Lab Chip 13, 3232–3238 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Kim SB et al. Super-absorbent polymer valves and colorimetric chemistries for time-sequenced discrete sampling and chloride analysis of sweat via skin-mounted soft microfluidics. Small 14, 1703334 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Sekine Y et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab Chip 18, 2178 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Kinnamon D, Ghanta R, Lin KC, Muthukumar S & Prasad S Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ciui B et al. Wearable wireless tyrosinase bandage and microneedle sensors: Toward melanoma screening. Adv. Healthcare Mater 7, 1701264 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Chen Y et al. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv 3, e1701629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lipani L et al. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol 13, 504–511 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Gibson LE & Cooke RE A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 23, 545–549 (1959). [PubMed] [Google Scholar]
- 90.Cole DEC & Boucher MJ Use of a new sample-collection device (macroduct) in anion analysis of human sweat. Clin. Chem 32, 1375–1378 (1986). [PubMed] [Google Scholar]
- 91.Matzeu G, Fay C, Vaillant A, Coyle S & Diamond D A wearable device for monitoring sweat rates via image analysis. IEEE Trans. Biomed. Eng 63, 1672–1680 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Tai LC et al. Methylxanthine drug monitoring with wearable sweat sensors. Adv. Mater 30 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Kim J et al. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Farandos NM, Yetisen AK, Monteiro MJ, Lowe CR & Yun SH Contact lens sensors in ocular diagnostics. Adv. Healthcare Mater 4, 792–810 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Baca JT, Finegold DN & Asher SA Tear glucose analysis for the noninvasive detection and monitoring of diabetes mellitus. Ocul. Surf 5, 280–293 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Thaysen JH & Thorn NA Excretion of urea, sodium, potassium and chloride in human tears. Am. J. Physiol 178, 160–164 (1954). [DOI] [PubMed] [Google Scholar]
- 97.Mitsubayashi K & Arakawa T Cavitas sensors: Contact lens type sensors & mouthguard sensors. Electroanalalysis 28, 1170–1187 (2016). [Google Scholar]
- 98.Pankratov D, Gonzalez-Arribas E, Blum Z & Shleev S Tear based bioelectronics. Electroanalalysis 28, 1250–1266 (2016). [Google Scholar]
- 99.Sen DK & Sarin GS Tear glucose-levels in normal people and in diabetic-patients. Br. J. Ophthalmol 64, 693–695 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chu MX et al. Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta 83, 960–965 (2011). [DOI] [PubMed] [Google Scholar]
- 101.Chu MX et al. Biomedical soft contact-lens sensor for in situ ocular biomonitoring of tear contents. Biomed. Microdevices 13, 603–611 (2011). [DOI] [PubMed] [Google Scholar]
- 102.Mishima S, Gasset A, Klyce SD Jr. & Baum JL Determination of tear volume and tear flow. Invest. Ophthalmol 5, 264–276 (1966). [PubMed] [Google Scholar]
- 103.Fullard RJ & Carney LG Diurnal variation in human tear enzymes. Exp. Eye Res 38, 15–26 (1984). [DOI] [PubMed] [Google Scholar]
- 104.Yan Q et al. Measurement of tear glucose levels with amperometric glucose biosensor/capillary tube configuration. Anal. Chem 83, 8341–8346 (2011). [DOI] [PubMed] [Google Scholar]
- 105.Stuchell RN, Feldman JJ, Farris RL & Mandel ID The effect of collection technique on tear composition. Invest. Ophthalmol. Vis. Sci 25, 374–377 (1984). [PubMed] [Google Scholar]
- 106.Green-Church KB, Nichols KK, Kleinholz NM, Zhang LW & Nichols JJ Investigation of the human tear film proteome using multiple proteomic approaches. Mol. Vis 14, 456–470 (2008). [PMC free article] [PubMed] [Google Scholar]
- 107.Alexeev VL, Das S, Finegold DN & Asher SA Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin. Chem 50, 2353–2360 (2004). [DOI] [PubMed] [Google Scholar]
- 108.Ben-Moshe M, Alexeev VL & Asher SA Fast responsive crystalline colloidal array photonic crystal glucose sensors. Anal Chem 78, 5149–5157 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Domschke A, March WF, Kabilan S & Lowe C Initial clinical testing of a holographic non-invasive contact lens glucose sensor. Diabetes Technol. Ther 8, 89–93 (2006). [DOI] [PubMed] [Google Scholar]
- 110.Khalil OS Noninvasive photonic-crystal material for sensing glucose in tears. Clin. Chem 50, 2236–2237 (2004). [DOI] [PubMed] [Google Scholar]
- 111.Cai ZY, Smith NL, Zhang JT & Asher SA Two-dimensional photonic crystal chemical and biomolecular sensors. Anal. Chem 87, 5013–5025 (2015). [DOI] [PubMed] [Google Scholar]
- 112.Elsherif M, Hassan MU, Yetisen AK & Butt H Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 12, 5452–5462 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sung YL, Campa F & Shih WC Open-source do-it-yourself multi-color fluorescence smartphone microscopy. Biomed. Opt. Express 8, 5075–5086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yao H, Afanasiev A, Lahdesmaki I & Parviz BA A dual microscale glucose sensor on a contact lens, tested in conditions mimicking the eye. Proc. IEEE Micro Electro Mech. Syst, 25–28 (2011). [Google Scholar]
- 115.Yao H et al. A contact lens with integrated telecommunication circuit and sensors for wireless and continuous tear glucose monitoring. J. Micromech. Microeng 22, 075007 (2012). [Google Scholar]
- 116.Liao YT, Yao HF, Lingley A, Parviz B & Otis BP A 3-μW CMOS glucose sensor for wireless contact-lens tear glucose monitoring. IEEE J. Solid-State Circuits 47, 335–344 (2012). [Google Scholar]
- 117.Kim J et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun 8, 14997 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park J et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv 4, eaap9841 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kownacka AE et al. Clinical evidence for use of a non-invasive biosensor for tear glucose as an alternative to painful finger-prick for diabetes management utilizing biopolymer coating. Biomacromolecules (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zubareva T & Kiseleva Z Catecholamine content of the lacrimal fluid of healthy people and glaucoma patients. Ophthalmologica 175, 339–344 (1977). [DOI] [PubMed] [Google Scholar]
- 121.Zhou L et al. In-depth analysis of the human tear proteome. J. Proteomics 75, 3877–3885 (2012). [DOI] [PubMed] [Google Scholar]
- 122.Pfaffe T, Cooper-White J, Beyerlein P, Kostner K & Punyadeera C Diagnostic potential of saliva: Current state and future applications. Clin. Chem 57, 675–687 (2011). [DOI] [PubMed] [Google Scholar]
- 123.Javaid MA, Ahmed AS, Durand R & Tran SD Saliva as a diagnostic tool for oral and systemic diseases. J. Oral Biol. Craniofacial Res 6, 66–75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goswami Y, Mishra R, Agrawal AP & Agrawal LA Salivary biomarkers: A review of powerful diagnostic tool. IOSR J. Dent. Med. Sci 14, 80–87 (2015). [Google Scholar]
- 125.Campuzano S, Yanez-Sedeno P & Pingarron JM Electrochemical bioaffinity sensors for salivary biomarkers detection. Trends Anal. Chem 86, 14–24 (2017). [Google Scholar]
- 126.Soni A & Jha SK A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens. Bioelectron 67, 763–768 (2015). [DOI] [PubMed] [Google Scholar]
- 127.Stevens RC, Soelberg SD, Near S & Furlong CE Detection of cortisol in saliva with a flow-filtered, portable surface plasmon resonance biosensor system. Anal. Chem 80, 6747–6751 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamaguchi M et al. Hand-held monitor of sympathetic nervous system using salivary amylase activity and its validation by driver fatigue assessment. Biosens. Bioelectron 21, 1007–1014 (2006). [DOI] [PubMed] [Google Scholar]
- 129.Ballesta Claver J, Valencia Miron MC & Capitan-Vallvey LF Disposable electrochemiluminescent biosensor for lactate determination in saliva. Analyst 134, 1423–1432 (2009). [DOI] [PubMed] [Google Scholar]
- 130.Zangheri M et al. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron 64, 63–68 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Mahosenaho M et al. A disposable biosensor for the determination of alpha-amylase in human saliva. Microchim. Acta 170, 243–249 (2010). [Google Scholar]
- 132.Soni A & Jha SK Smartphone based non-invasive salivary glucose biosensor. Anal. Chim. Acta 996, 54–63 (2017). [DOI] [PubMed] [Google Scholar]
- 133.Zhang L, Yang WT, Yang YK, Liu H & Gu ZZ Smartphone-based point-of-care testing of salivary alpha-amylase for personal psychological measurement. Analyst 140, 7399–7406 (2015). [DOI] [PubMed] [Google Scholar]
- 134.Roda A et al. A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst 139, 6494–6501 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Aguirre A et al. Sialochemistry - a diagnostic-tool. Crit. Rev. Oral Biol. Med 4, 343–350 (1993). [DOI] [PubMed] [Google Scholar]
- 136.Kaufman E & Lamster IB The diagnostic applications of saliva - a review. Crit. Rev. Oral Biol. Med 13, 197–212 (2002). [DOI] [PubMed] [Google Scholar]
- 137.Chiappin S, Antonelli G, Gatti R & De Palo EF Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 383, 30–40 (2007). [DOI] [PubMed] [Google Scholar]
- 138.Streckfus CF & Bigler LR Saliva as a diagnostic fluid. Oral Dis. 8, 69–76 (2002). [DOI] [PubMed] [Google Scholar]
- 139.Mandel ID The diagnostic uses of saliva. J. Oral Pathol. Med 19, 119–125 (1990). [DOI] [PubMed] [Google Scholar]
- 140.Malon RSP, Sadir S, Balakrishnan M & Corcoles EP Saliva-based biosensors: Noninvasive monitoring tool for clinical diagnostics. Biomed. Res. Int 2014, 962903 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chicharro JL, Lucia A, Perez M, Vaquero AF & Urena R Saliva composition and exercise. Sports Med. 26, 17–27 (1998). [DOI] [PubMed] [Google Scholar]
- 142.Singh M Non invasive diagnostic tool for pathological conditions salivary biomarkers. Int. J. Pharm. Biol. Arch 5, 1–12 (2014). [Google Scholar]
- 143.Gatti R & De Palo EF An update: Salivary hormones and physical exercise. Scand. J. Med. Sci. Sports 21, 157–169 (2011). [DOI] [PubMed] [Google Scholar]
- 144.Viswanath B, Choi CS, Lee K & Kim S Recent trends in the development of diagnostic tools for diabetes mellitus using patient saliva. Trends Anal. Chem 89, 60–67 (2017). [Google Scholar]
- 145.Kim J et al. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 139, 1632–1636 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Segura R et al. A new approach to the assessment of anaerobic metabolism: Measurement of lactate in saliva. Br. J. Sports Med 30, 305–309 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Santos RVT, Almeida ALR, Caperato EC, Martins E & Costa Rosa LFBP Effects of a 30-km race upon salivary lactate correlation with blood lactate. Comp. Biochem. Physiol. B: Biochem. Mol. Biol 145, 114–117 (2006). [DOI] [PubMed] [Google Scholar]
- 148.Arakawa T et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron 84, 106–111 (2016). [DOI] [PubMed] [Google Scholar]
- 149.Yamaguchi M, Mitsumori M & Kano Y Noninvasively measuring blood glucose using saliva. IEEE Eng. Med. Biol. Mag 17, 59–63 (1998). [DOI] [PubMed] [Google Scholar]
- 150.Agrawal R et al. Noninvasive method for glucose level estimation by saliva. J Diabetes Metab. 4, 2–5 (2013). [Google Scholar]
- 151.Zhang W, Du Y & Wang ML Noninvasive glucose monitoring using saliva nano-biosensor. Sens. Bio-Sens. Res 4, 23–29 (2015). [Google Scholar]
- 152.Jurysta C et al. Salivary glucose concentration and excretion in normal and diabetic subjects. J. Biomed. Biotechnol 2009, 6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Campbell MJA Glucose in the saliva of the non-diabetic and the diabetic patient. Arch. Oral Biol 10, 197–205 (1965). [DOI] [PubMed] [Google Scholar]
- 154.Abikshyeet P, Ramesh V & Oza N Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes Metab. Syndrome Obesity: Targets Ther 5, 149–154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tseng P, Napier B, Garbarini L, Kaplan DL & Omenetto FG Functional, rf-trilayer sensors for tooth-mounted, wireless monitoring of the oral cavity and food consumption. Adv. Mater 30 (2018). [DOI] [PubMed] [Google Scholar]
- 156.Lee Y et al. Wireless, intraoral hybrid electronics for real-time quantification of sodium intake toward hypertension management. P. Natl. Acad. Sci. U.S.A 115, 5377–5382 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sarpeshkar R Universal principles for ultra low power and energy efficient design. IEEE Trans. Circuits Syst. II 59, 193–198 (2012). [Google Scholar]
- 158.De Marcellis A, Depari A, Ferri G, Flammini A & Sisinni E A CMOS integrated low-voltage low-power time-controlled interface for chemical resistive sensors. Sens. Actuators B 179, 313–318 (2013). [Google Scholar]
- 159.Zhang Y et al. Realizing both high energy and high power densities by twisting three carbon-nanotube-based hybrid fibers. Angew. Chem. Int. Ed 54, 11177–11182 (2015). [DOI] [PubMed] [Google Scholar]
- 160.Huang Y et al. Weavable, conductive yarn-based NiCo//Zn textile battery with high energy density and rate capability. ACS Nano 11, 8953–8961 (2017). [DOI] [PubMed] [Google Scholar]
- 161.Mesin L A neural algorithm for the non-uniform and adaptive sampling of biomedical data. Comp. Biol. Med 71, 223–230 (2016). [DOI] [PubMed] [Google Scholar]
- 162.Zi Y et al. Triboelectric–pyroelectric–piezoelectric hybrid cell for high-efficiency energy-harvesting and self-powered sensing. Adv. Mater 27, 2340–2347 (2015). [DOI] [PubMed] [Google Scholar]
- 163.Zhang K & Yang Y Thermo-phototronic effect enhanced InP/ZnO nanorod heterojunction solar cells for self-powered wearable electronics. Adv. Funct. Mater 27, 1703331 (2017). [Google Scholar]
- 164.Wen Z et al. Self-powered textile for wearable electronics by hybridizing fiber-shaped nanogenerators, solar cells, and supercapacitors. Sci. Adv 2, e1600097 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bandodkar AJ et al. Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat. Enegry Env. Sci 10, 1581–1589 (2017). [Google Scholar]
- 166.Ha M, Park J, Lee Y & Ko H Triboelectric generators and sensors for self-powered wearable electronics. ACS Nano 9, 3421–3427 (2015). [DOI] [PubMed] [Google Scholar]
- 167.Kim CS et al. Self-powered wearable electrocardiography using a wearable thermoelectric power generator. ACS Energy Lett., 501–507 (2018). [Google Scholar]
- 168.Imani S, Mercier PP, Bandodkar AJ, Kim J & Wang J in Circuits and Systems (ISCAS), 2016 IEEE International Symposium on 1122-1125 (IEEE, 2016). [Google Scholar]
- 169.Khan SU, Zomaya AY & Abbas A Handbook of large-scale distributed computing in smart healthcare. (Springer, 2017). [Google Scholar]
- 170.Li M, Lou W & Ren K Data security and privacy in wireless body area networks. IEEE Wireless communications 17 (2010). [Google Scholar]
- 171.Kotz D, Gunter CA, Kumar S & Weiner JP Privacy and security in mobile health: a research agenda. Computer 49, 22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dimitriou T & Ioannis K in Applied Sciences on Biomedical and Communication Technologies, 2008. ISABEL'08. First International Symposium on 1-5 (IEEE, 2008). [Google Scholar]
- 173.Daniels JS & Pourmand N Label-free impedance biosensors: Opportunities and challenges. Electroanalalysis 19, 1239–1257 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xiao Y, Lubin AA, Heeger AJ & Plaxco KW Label-free electronic detection of thrombin in blood serum by using an aptamer-based sensor. Angew. Chem. Int. Ed 44, 5456–5459 (2005). [DOI] [PubMed] [Google Scholar]
- 175.Hammami A, Raymond N & Armand M Lithium ion batteries: Runaway risk of forming toxic compounds. Nature 424, 635–636 (2003). [DOI] [PubMed] [Google Scholar]
- 176.Lisbona D & Snee T A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf Environ 89, 434–442 (2011). [Google Scholar]
- 177.Xu S et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun 4 (2013). [DOI] [PubMed] [Google Scholar]
- 178.Berchmans S et al. An epidermal alkaline rechargeable Ag-Zn printable tattoo battery for wearable electronics. J. Mater. Chem. A 2, 15788–15795 (2014). [Google Scholar]
- 179.Kumar R et al. All-printed, stretchable Zn-Ag2O rechargeable battery via hyperelastic binder for self-powering wearable electronics. Adv Energy Mater 7 (2017). [Google Scholar]
- 180.Qiu YC et al. Vertically aligned carbon nanotubes on carbon nanofibers: A hierarchical three-dimensional carbon nanostructure for high-energy flexible supercapacitors. Chem. Mater 27, 1194–1200 (2015). [Google Scholar]
- 181.Li Q, Lu XF, Xu H, Tong YX & Li GR Carbon/MnO2 double-walled nanotube arrays with fast ion and electron transmission for high-performance supercapacitors. ACS Appl. Mater. Interfaces 6, 2726–2733 (2014). [DOI] [PubMed] [Google Scholar]
- 182.Wang Q, Yan J & Fan ZJ Carbon materials for high volumetric performance supercapacitors: Design, progress, challenges and opportunities. Enegry Env. Sci 9, 729–762 (2016). [Google Scholar]
- 183.O'Connor TF et al. Wearable organic solar cells with high cyclic bending stability: Materials selection criteria. Sol Energ Mat Sol C 144, 438–444 (2016). [Google Scholar]
- 184.Kim BJ et al. Highly efficient and bending durable perovskite solar cells: Toward a wearable power source. Enegry Env. Sci 8, 916–921 (2015). [Google Scholar]
- 185.Yang JH et al. Effect of garment design on piezoelectricity harvesting from joint movement. Smart Mater Struct 25 (2016). [Google Scholar]
- 186.Pu X et al. Wearable self-charging power textile based on flexible yarn supercapacitors and fabric nanogenerators. Adv. Mater 28, 98–105 (2016). [DOI] [PubMed] [Google Scholar]
- 187.Oh JY et al. Chemically exfoliated transition metal dichalcogenide nanosheet-based wearable thermoelectric generators. Enegry Env. Sci 9, 1696–1705 (2016). [Google Scholar]
- 188.Lu ZS, Zhang HH, Mao CP & Li CM Silk fabric-based wearable thermoelectric generator for energy harvesting from the human body. Appl Energ 164, 57–63 (2016). [Google Scholar]
- 189.Jia WZ, Valdes-Ramirez G, Bandodkar AJ, Windmiller JR & Wang J Epidermal biofuel cells: Energy harvesting from human perspiration. Angew. Chem. Int. Ed 52, 7233–7236 (2013). [DOI] [PubMed] [Google Scholar]
- 190.Jia WZ et al. Wearable textile biofuel cells for powering electronics. J. Mater. Chem. A 2, 18184–18189 (2014). [Google Scholar]
- 191.Bandodkar AJ & Wang J Wearable biofuel cells: A review. Electroanalalysis 28, 1188–1200 (2016). [Google Scholar]
- 192.Ogawa Y et al. Stretchable biofuel cell with enzyme-modified conductive textiles. Biosens. Bioelectron 74, 947–952 (2015). [DOI] [PubMed] [Google Scholar]
- 193.Falk M, Andoralov V, Silow M, Toscano MD & Shleev S Miniature biofuel cell as a potential power source for glucose-sensing contact lenses. Anal. Chem 85, 6342–6348 (2013). [DOI] [PubMed] [Google Scholar]
- 194.Grattieri M & Minteer SD Self-powered biosensors. ACS Sens. 3, 44–53 (2018). [DOI] [PubMed] [Google Scholar]
- 195.Yetisen AK, Martinez-Hurtado JL, Garcia-Melendrez A, Vasconcellos FD & Lowe CR A smartphone algorithm with inter-phone repeatability for the analysis of colorimetric tests. Sens. Actuators B 196, 156–160 (2014). [Google Scholar]