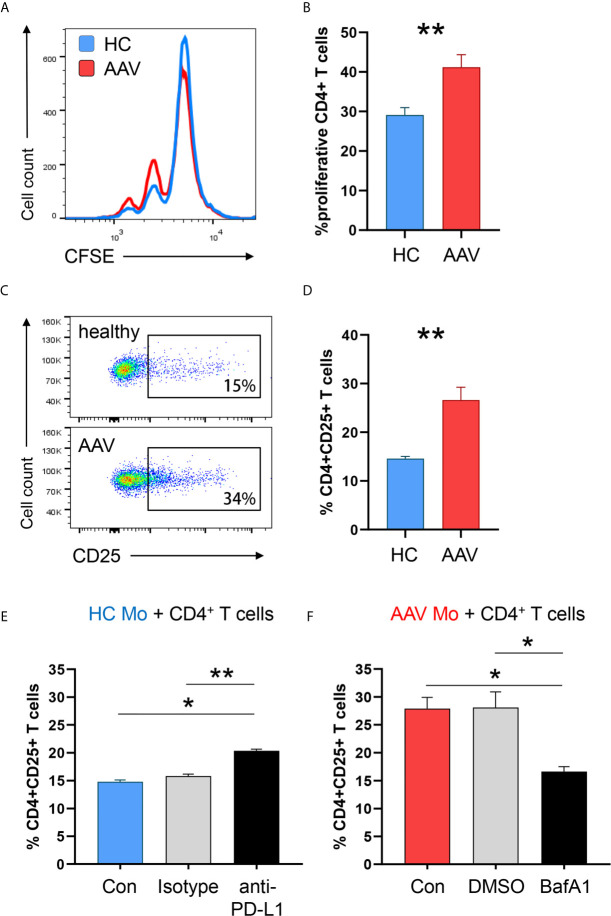
Figure 7.
PD-L1lo monocytes from AAV patients show enhanced stimulatory capacity. Monocytes from healthy or AAV donors were pretreated with IFNγ for 24h. Then, their capacity to stimulate T cells was probed by coculturing them with CD4+ T cells from healthy donors (ratio monocytes/T cells 1:3). T-cell proliferation was determined through CFSE dilution and T-cell activation was quantified by the frequency of CD4+CD25+ T cells. (A) Proliferation of CD4+ T cells was measured by flow cytometry after 5 days of co-culture. Representative histograms of CSFE expression. (B) Frequencies of proliferating CD4+ T cells when cocultured with either HC or AAV patient-derived monocytes (n=8 each group). (C) Activated CD4+CD25+ T cells quantified by flow cytometry after 48 h. (D) Percentage of activated CD4+ T cells after coculture (n=6 each group). (E) Co-culture with monocytes from healthy donors was performed in the presence of anti-PD-L1 antibodies or isotype control. Frequencies of activated CD4+CD25+ T cells from 6 independent experiments (isotype n=3) were measured after 48 h by flow cytometry. (F) Co-culture with monocytes from AAV patients was performed after monocytes were pre-treated with the lysosomal inhibitor BafA1 (20nM) or DMSO vector control. Frequencies of activated CD4+CD25+ T cells from 6 independent experiments (DMSO n=3) were measured after 48 h by flow cytometry. Mann-Whitney test (B), unpaired t-test (D) and Kruskal-Wallis test with Dunn’s multiple comparisons test (E, F) were applied. *P<0.05; **P<0.01. Bar graph shows mean ± SEM. Bar graph shows mean ± SEM. AAV, ANCA-associated vasculitis; HC, healthy control donors; MFI, mean fluorescence intensity; PD-L1, Programmed death-ligand 1; M-CSF, macrophage colony-stimulating factor; GM-CSF, Granulocyte-macrophage colony-stimulating factor.