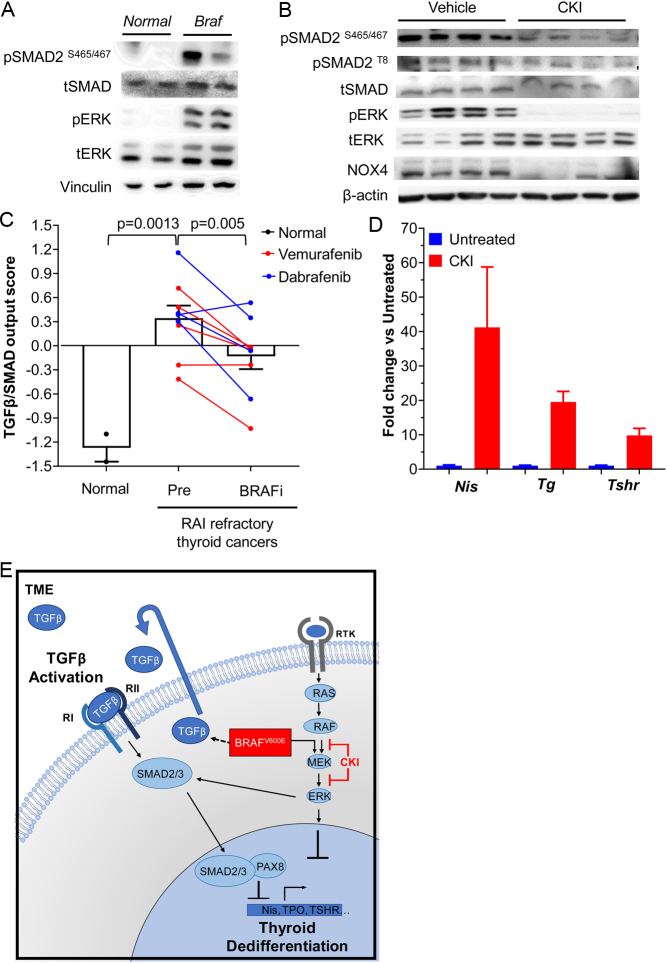
Figure 1.
SMAD pathway activation in BRAFV600E-induced PTCs. (A) Western blot of normal thyroid and tumor lysates from TPO-Cre/LSL-BrafV600E (Braf) mice probed with antibodies to the indicated proteins. (B) Western blot of tumor tissues from Braf mice treated with vehicle or the MEK inhibitor CKI127 (CKI) probed for the indicated targets. Each lane corresponds to an individual mouse PTC (n = 4 per group). (C) TGFβ-SMAD transcriptional output scores of normal thyroid tissues compared to biopsy samples from patients with RAI-refractory thyroid cancers taken prior to and while on treatment with the RAF kinase inhibitors dabrafenib (blue) or vemurafenib (red) for 2 weeks. Each line depicts paired biopsy results from the same lesion prior to and while on drug. (D) Quantitative RT-PCR of thyroid differentiation markers in tumors from panel B. (E) Interactions between the MAPK pathway and TGFβ signaling in thyroid cancer. Oncogenic BRAF induces dedifferentiation in part by ERK-induced silencing or inactivation of lineage transcription factors and by interfering with TSH-induced cAMP signaling (Mitsutake et al. 2005) (not shown). BRAFV600E also increases tumor cell secretion of TGFB1, leading to SMAD impairment of transactivation of thyroid-specific genes by the lineage transcription factor PAX8. Oncogenic BRAF also induces pERK phosphorylation at the T8 residue of SMAD, promoting its additional phosphorylation and activation by the TGFB1 receptor.

 This work is licensed under a
This work is licensed under a