Supplemental Digital Content is available in the text.
Background.
To improve the measurement of organ procurement organization (OPO) performance, the Center for Medicare and Medicaid Services recently proposed using inpatient deaths defined as the eligible pool of organ donors within an OPO as patients 75 years or younger that died from any cause that would not preclude donation.
Methods.
To account for the geographic variation in OPO performance and organ availability across the United States, we utilized spatial analysis to appraise the newly proposed metric of inpatient deaths.
Results.
Using spatial clustering that accounts for geographic relationships between Organ Procurement Organizations, the top 5 causes of donation-eligible death, and inpatient deaths, we identified 4 unique OPO clusters. Each group had a distinct demographic composition, cause of death, and inpatient death pattern. In multivariate analysis accounting for these geographic relationships, the spatial clusters remained significantly associated with the outcome of inpatient deaths (P < 0.001) and were the best-fitting model compared with models without the spatial clusters; this suggests that further risk adjustment of inpatient deaths should include these geographic considerations.
Conclusions.
This approach provides not only a manner to assess donor potential by improving risk adjustment but also an opportunity to further explore geographic and spatial relationships in the practice of organ transplantation and OPO performance.
INTRODUCTION
In the United States, the Organ Procurement Organizations (OPOs) are responsible for the evaluation and procurement of deceased donor organs within their assigned donation service areas (DSAs). Eligible deaths, the current common denominator for many OPO performance metrics,1 are vulnerable to selective reporting and therefore biasing the results.2 The consequences of a self-reported measure that is underinclusive of true potential are only compounded by the differences between the OPOs: OPO performance is further influenced by differences in donor availability,3,4 DSA geography and demographics,5 and organ acceptance patterns of transplant centers in neighboring DSAs.6,7 Many of these are consequences of the geographic borders and locally oriented organ allocation.
To address these shortcomings, the 2019 Executive Order on Advancing American Kidney Health8 contained a mandate to improve OPO performance metrics. In response, the Centers for Medicare and Medicaid Services (CMS) proposed “inpatient deaths” to replace eligible deaths by changing “the OPO donation rate measure to the number of organ donors in the OPO’s service area as a percentage of inpatient deaths among patients 75 years old or younger from any cause of death that would not prevent donation (eg, organs from those with metastatic cancer and a recent history of cancer cannot be transplanted).”9 As with any new metric that would have wide-ranging effects, further evaluation with both traditional and novel methods is essential.
Geography, conceived as both the geographic borders that define organ allocation as well as the populations, resources, and infrastructure that these spaces contain, influences organ allocation and the practice of transplantation. It has been studied in access to transplantation,10,11 local measures of socioeconomic status,12 and spatial organization of transplant centers.13,14 Donation rates are also influenced by geography and special characteristics, from geographic variation between state policies encouraging organ donation,15 local variation in donor registration,16 and social capital.17 Prior spatial analysis specific to OPOs reinforced the poor validity of eligible deaths: Cannon et al demonstrated spatial autocorrelation of potentially donation-eligible mortality patterns that was not observed for eligible deaths, but the analysis was not advanced to incorporate a spatial regression approach.18 Extending these spatial analytic principles, accounting for the fact that certain population characteristics of geographically proximate OPOs are more similar than OPOs that are further apart (Tobler’s first law of geography19 and as demonstrated in Cannon et al18) could lead to a better understanding of the geographic variability in possible organ donors and therefore lead to increased deceased organ donation. The application of spatial analysis has potential applications as allocation shifts away from the OPO territories and to broader sharing.
Here, we aim to utilize spatial analysis to better understand and to appraise the CMS proposal of inpatient deaths. First, we explore the spatial relationships and patterns between the OPOs, causes of donation-eligible deaths, and inpatient deaths. We then extend the work of Canon et al by modeling these in a bivariate fashion to determine if spatial analysis is appropriate. Next, we use clustering to create groups of similar OPOs to adjust the number of inpatient deaths, understanding their varying levels of donor potential, by accounting for the additional information available from geographic analysis based on its constituent parts. This approach to analysis would enable an assessment of how cause of death, the racial and ethnic demographics, and geography overlap and contribute to organ donation in the United States. Ultimately, this work aims to demonstrate how spatial relationships affect inpatient deaths and to argue for future spatial analysis in setting transplant performance metrics.
METHODS
Data Sources
Eligible deaths, OPO demographic characteristics, and a list of counties served by each OPO was obtained from the January 2018 OPO Specific Reports from the Scientific Registry of Transplant Recipients.1 This served as the source for eligible deaths for 2016 for each OPO, which was used for comparison to the prior work of Cannon et al.18
Matching prior work,18 the top 5 causes of death among organ donors (gunshot, blunt trauma, overdose, cerebrovascular disease, and cardiovascular disease) were aggregated from counties using CDC WONDER20 from 2014 to 2016.20 To estimate the number of inpatient deaths according to the CMS proposal, we followed the CDC WONDER query of Snyder et al covering 2014 to 2016.21 In CDC WONDER, deaths are attributed to the home county of the decedent, not where death occurs, and data are not available to assign deaths to the counties in which they occur.
These data were derived from the following resources available in the public domain: OPO-specific reports (https://www.srtr.org/reports-tools/opo-specific-reports/) and CDC WONDER (https://wonder.cdc.gov/).
Statistical Analysis
Organ Donor Center of Hawaii (HIOP) was excluded due to distance affecting spatial weights, and Life Link of Puerto Rico (PRLL) was excluded due to missing death cause data; this left 56 OPOs for analysis. Moran’s I (using queen continuity)22 assessed spatial autocorrelation and identified geographic clusters of similar inpatient and cause-specific death rates. Bivariate analyses, measuring the spatial association between the inpatient death rate and each of the cause-specific death rates separately, were conducted to further understand the relationship and identify areas of geographic variability using bivariate local Moran’s I.23
After establishing both univariate (global) spatial dependencies with Moran’s I and bivariate (local) spatial dependencies with bivariate local Moran’s I, we executed a clustering procedure using all those 6 variables to investigate existence of clusters. To do that, we have considered 3 different clustering procedures, and then they were compared based on the tool called “within cluster sums of squares” (WCSOS); and the final clustering procedure will be selected based on smallest value of WCSOS. Here, we have considered 3 well-known methods: K-means clustering, hierarchical clustering, and ClustGeo,24 a hierarchical clustering with spatial constraints within R. We chose to proceed with ClustGeo as it produced smallest WCSOS compared with 2 other clustering procedure.
Because ClustGeo contains spatial constraints and was the best-fitting model, we elected to use this method to account for all relevant variables, including the spatial clustering and causes of death consistent with organ donation. As ClustGeo incorporates spatial information in the clustering method, we find that it can be useful to use in the models to develop clusters among the OPOs. In this clustering procedure, for comparison of magnitude of relative contributions, we have standardized all the variables: by subtracting the group mean and dividing by the SD, giving each measure a mean of 0 and a SD of 1.
In the next phase, we used a multivariable linear regression procedure to examine the association between the inpatient death rates, the top 5 causes of death among organ donors, and the clustering variable. This regression model was also adjusted for other demographic variables of the OPOs. Given the large number of covariates and limited number of observations, a stepwise regression procedure was used to find the final parsimonious model, selected by Akaike Information Criterion. Further descriptions of the methods are included in the Appendix (SDC, http://links.lww.com/TXD/A305).
A P value <0.05 was used as criteria for statistical significance. No adjustments were made for multiple comparisons. All statistical analyses and data linkages were performed using R 4.0.2 (R Core Team, Vienna, Austria). Basic spatial analysis was conducted with GeoDa 1.14,25 and clustering was performed with the R package ClustGeo.24 Maps were made with QGIS 3.12.1 (QGIS Development Team, Open Source Geospatial Foundation Project, http://qgis.osgeo.org).
IRB Approval and Data Access
The Institutional Review Board of Partners Healthcare approved this study under a human subjects exemption, as it uses publicly available data. The complied data that support the findings of this study are available in Harvard Dataverse at https://doi.org/10.7910/DVN/J1A4KS.26
RESULTS
Establishment of Spatial Autocorrelation
First, we explored basic univariate measures of global spatial autocorrelation. The eligible death rate (per 100 000 population) was first mapped for each OPO (Figure 1A), which did not show significant spatial autocorrelation (Moran’s I = 0.14, P = 0.109). The lack of autocorrelation indicates a random association of eligible death rates among the OPOs, meaning that there is no spatial relationship in the data. The inpatient death rate (per 100 000 population) was then mapped (Figure 1B); here, significant spatial autocorrelation was observed (Moran’s I = 0.54, P = 0.001), which suggests that the data are subject to spatial analysis. Of note, the difference between eligible death rates and inpatient death rates was nearly 100-fold.
FIGURE 1.
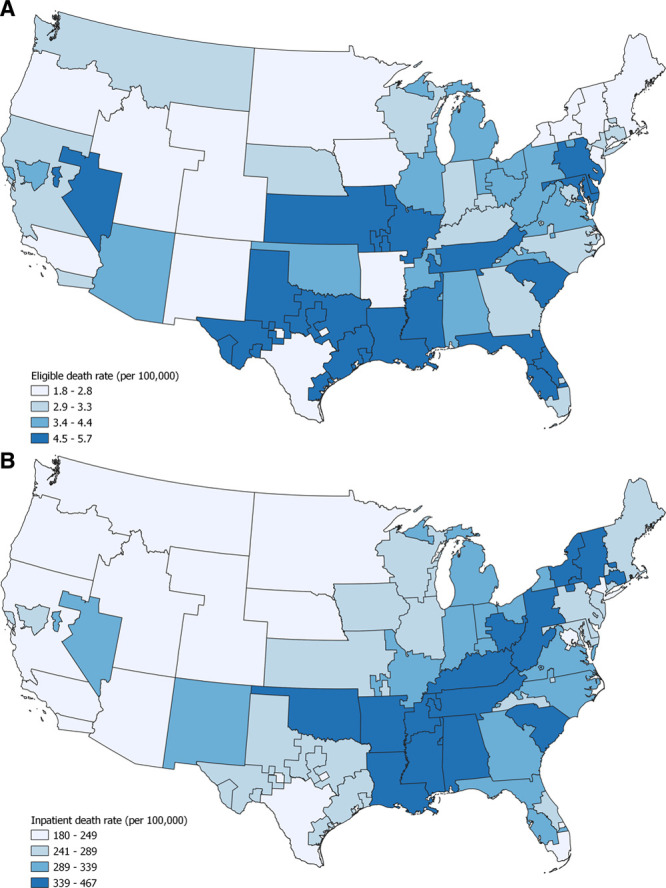
Eligible death rate per 100 000 OPO population in (A) and inpatient death rate per 100 000 OPO population (B). There was no significant spatial autocorrelation for the eligible death rate (Moran’s I = 0.14, P = 0.09), but there was an association for the inpatient death rate (Moran’s I = 0.54, P = 0.001). Both maps are in quartiles; inpatient death rates are approximately 100 times higher. Eligible death rates were obtained from the OPO Specific Reports of the Scientific Registry of Transplant Recipients; inpatient death rates were derived from a query of CDC WONDER. Map created with QGIS 3.12.1.
Next, we moved from univariate to the bivariate analysis, using bivariate local Moran’s I. This measurement identifies OPOs that have a high inpatient death rate and are surrounded by those with either a low- or high cause–specific death rate (eg, gunshot wounds), as well as those with a low inpatient death rate and surrounded by a low- or high cause–specific death rate (Figure 2A–E). This demonstrated significant associations between all 4 types of outputs: high inpatient death rates with both high and low cause-specific death rates, as well as lower inpatient death rates that had both high and low cause-specific death rates (P < 0.05 for all associations). The OPOs of high inpatient death rates generally had higher component death rates except for overdose deaths (Figure 2A). For OPOs with lower inpatient death rates, there are a number of high component death rates compared with lower inpatient death rates for overdose (Figure 2A), gunshot (Figure 2B), and blunt trauma (Figure 2C).
FIGURE 2.
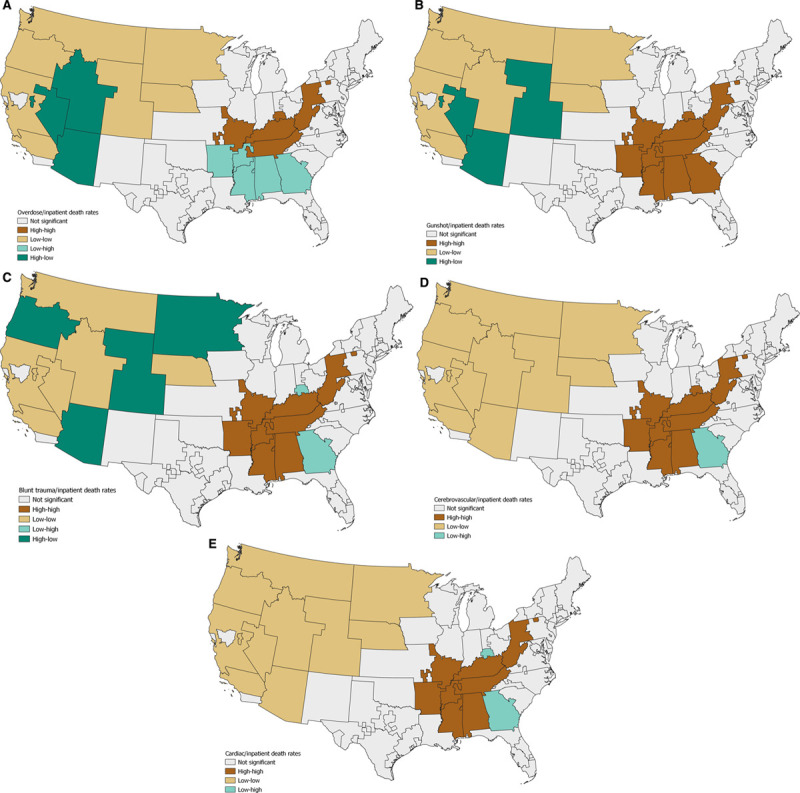
Bivariate local Moran’s I identified OPOs where the cause-specific death rate is associated with the inpatient death rate, both by location and magnitude of the death rates. For example, (A) this identifies OPOs of high-overdose death rates and high inpatient death rates (high-high, brown), high-overdose death rates and low inpatient death rates (high-low, tan), low-overdose death rates and high-inpatient death rates (low-high, teal), and low-overdose death rates and low inpatient death rates (low-low, dark-green). Different patterns were identified for gunshot wounds (B), blunt trauma (C), cerebrovascular (D), and cardiovascular (E) death rates. Maps created with QGIS 3.12.1.
Creation of the OPO Clusters
The clustering analysis created 4 groups based on the relationship between geography, the 5 causes of death, and inpatient death rates: group A had 7, B had 13, C had 27, and D had 9 OPOs (Table 1; individual OPO categorization available in data repository). Inspecting the standardized means (Figure 3A), there were multiple associations between different death rates and inpatient deaths. There was also a clear geographic pattern (Figure 3B).
TABLE 1.
Components of spatial clustering by grouping
| Death rates(per 100 000 OPO population) | Overall(n = 56) | Group A(n = 7) | Group B(n = 13) | Group C(n = 27) | Group D(n = 9) |
|---|---|---|---|---|---|
| Inpatient | 299.1 (8.99) | 425.1 (13.1)* | 224.4 (7.3)* | 290.2 (6.8) | 335.6 (6.3)* |
| Gunshot | 33.6 (1.52) | 49.1 (2.9)* | 27.2 (2.3)* | 33.7 (2.1) | 30.3 (3.1) |
| Blunt trauma | 75.7 (2.44) | 90.2 (3.8)* | 67.0 (4.1)* | 80.0 (4.0) | 76.3 (4.6) |
| Overdose | 53.3 (3.03) | 49.4 (7.0) | 35.6 (3.4)* | 55.1 (4.2) | 77.7 (6.4)* |
| Cerebrovascular disease | 135.1 (3.25) | 157.8 (5.0)* | 106.1 (3.7)* | 136.2 (3.7) | 156 (4.0)* |
| Cardiac disease | 619.9 (17.8) | 754.0 (16.3)* | 440.4 (13.7)* | 611.2 (10.7) | 801.0 (15.5)* |
Values are mean death rates (per 100 000 OPO population) of the components of the spatial clustering with standard errors presented parenthetically.
Asterisks indicate significant associations relative to the group mean (P < 0.05).
OPO, Organ Procurement Organization.
FIGURE 3.
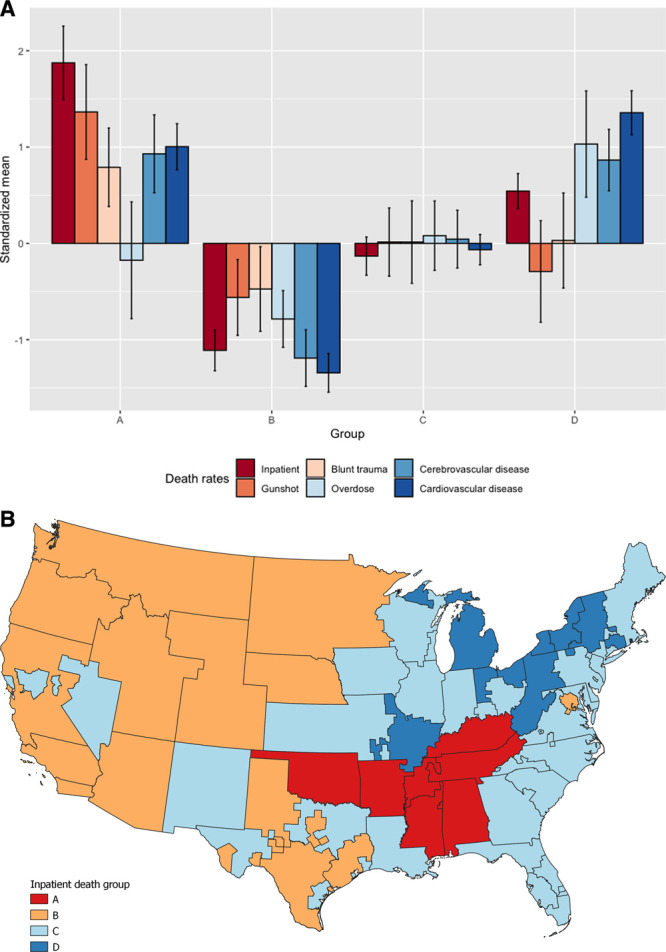
A, Standardized mean values of the component rates of the grouping. Errors indicate a 95% confidence interval. B, Clustered map based on the inpatient death rate for the 56 continental OPOs. The groups were created via a hierarchical spatial clustering method with the inpatient death rate and top 5 causes of donation-eligible death rates within each OPO. Map created with QGIS 3.12.1.
As noted in Table 1, the OPOs in group A were in the South, had the highest inpatient death rate, and were also significantly above average in gunshot, blunt trauma, cerebrovascular disease, and cardiovascular disease death rates. Group B was largely in the west and was significantly lower than the average in all rates, whereas group C was near the average for all rates and comprised OPOs in the Midwest and southeast. Group D, partially composed of the Rust Belt, was the second-highest inpatient death rate and was significantly higher in overdose, cerebrovascular disease, and cardiovascular disease death rates.
Examination of the OPO Characteristics
We then used the groupings to examine the basic characteristics among the OPOs (Table 2). The groups did not differ significantly based on total population or overall population density, but there was a higher overall death rate (all causes, not specific to organ donation) in groups A and D (P < 0.001). In terms of age distribution, group B had the youngest population (48.7% <35 y of age, P < 0.001), whereas group D had the oldest (30.9% 55 y of age or greater, P < 0.001). The race and ethnicity composition varied considerably, with group D being predominantly White (80.2%, P = 0.007), group A having the highest Black population (20.8%, P = 0.003), and group B containing the largest Hispanic population (7.3%, P < 0.001).
TABLE 2.
OPO characteristics by group
| Characteristic | Overall(n = 56) | Group A(n = 7) | Group B(n = 13) | Group C(n = 27) | Group D(n = 9) | P |
|---|---|---|---|---|---|---|
| Vital statistics | ||||||
| Total population | 5 786 317(495 039) | 3 708 165(535 874) | 7 655 625(1 324 352) | 5 964 312(675 814) | 4 168 560(820 220) | 0.06 |
| Population/square mile | 296.0 (63.6) | 91.4 (12.9) | 214.3 (100.9) | 410.7 (119.0) | 229.1 (46.2) | 0.34 |
| Deaths/1000 population | 8.8 (0.2) | 10.2 (0.1) | 7.1 (0.2) | 8.8 (0.2) | 10.0 (0.3) | <0.001 |
| Deaths/1000 square miles | 2.5 (0.5) | 0.9 (0.1) | 1.4 (0.6) | 3.4 (0.9) | 2.3 (0.5) | 0.20 |
| Demographic composition | ||||||
| Male (%) | 49.2 (0.1) | 48.9 (0.2) | 49.8 (0.1) | 49.1 (0.1) | 49.0 (0.1) | <0.001 |
| Age of population (%) | ||||||
| Under 10 | 12.5 (0.2) | 12.9 (0.2) | 13.5 (0.4) | 12.3 (0.2) | 11.3 (0.2) | <0.001 |
| 10–19 | 13.1 (0.1) | 13.3 (0.2) | 13.5 (0.3) | 12.9 (0.1) | 12.9 (0.2) | 0.15 |
| 20–34 | 20.5 (0.2) | 20.2 (0.2) | 21.7 (0.3) | 20.3 (0.2) | 19.5 (0.2) | <0.001 |
| 35–44 | 12.5 (0.1) | 12.4 (0.1) | 13.1 (0.2) | 12.5 (0.1) | 11.5 (0.1) | <0.001 |
| 45–54 | 13.4 (0.1) | 13.1 (0.2) | 12.8 (0.2) | 13.5 (0.1) | 13.8 (0.1) | 0.001 |
| 55–64 | 12.9 (0.1) | 12.8 (0.1) | 12.0 (0.3) | 12.9 (0.1) | 14.1 (0.2) | <0.001 |
| 65–74 | 8.8 (0.1) | 9.0 (0.2) | 7.8 (0.3) | 8.9 (0.2) | 9.4 (0.2) | <0.001 |
| 75–84 | 4.5 (0.1) | 4.6 (0.1) | 3.9 (0.2) | 4.6 (0.1) | 5.0 (0.1) | <0.001 |
| Over 85 | 2.0 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 2.0 (0.1) | 2.4 (0.1) | <0.001 |
| Race/ethnicity (%) | ||||||
| White | 65.6 (2.1) | 68.5 (4.8) | 58.3 (5.2) | 63.8 (2.8) | 80.2 (1.8) | 0.007 |
| Black | 12.6 (1.3) | 20.8 (5.6) | 6.5 (1.9) | 14.4 (1.6) | 9.7 (1.1) | 0.003 |
| Asian | 2.0 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 2.0 (0.1) | 2.4 (0.1) | <0.001 |
| Hispanic | 14.4 (1.8) | 5.4 (1.0) | 24.7 (4.3) | 14.7 (2.3) | 5.3 (1.3) | <0.001 |
| Other | 5.4 (0.2) | 2.1 (1.0) | 7.3 (0.3) | 5.1 (0.3) | 2.4 (0.1) | 0.40 |
Values are presented as the mean with standard errors in parentheses. P are from a 1-way ANOVA.
ANOVA, analysis of variance; OPO, organ procurement organization.
Regression for Inpatient Deaths Per Population
We then used these cluster groups to measure a covariate-adjusted association with inpatient deaths (Table 3). In the final stepwise regression (with all initial variables entered into the model), the groups were significantly associated with the inpatient death rate compared with group C, which was closest to the overall mean (A, 113.5 and P < 0.001; B, −47.1 and P < 0.001; D, 37.7 and P = 0.007). There was no association with the death rates of individual causes of death, as they were not included in the most parsimonious model. Among the age distribution, the only group with a significant association (in either direction) was ages 35–44 (−35.2 per percentage point increase, P < 0.001).
TABLE 3.
Multivariable linear regression for inpatient death rate per 100 000 for each OPO
| Covariate | Effect estimate | P |
|---|---|---|
| Group (C as reference) | ||
| Group A | 113.5 (12.0) | <0.001 |
| Group B | −47.1 (12.3) | <0.001 |
| Group D | 37.7 (13.4) | 0.007 |
| Race/ethnicity distribution (percent) | ||
| White | −0.9 (0.4) | 0.01 |
| Asian | −133.8 (32.8) | <0.001 |
| Other | −6.8 (2.7) | 0.02 |
| Age distribution (percent) | ||
| Under 10 | −29.3 (21.9) | 0.19 |
| 10–19 | −23.0 (14.7) | 0.13 |
| 20–34 | −28.0 (15.0) | 0.07 |
| 35–44 | −52.4 (13.8) | <0.001 |
| 45–54 | −35.2 (26.1) | 0.18 |
| 65–74 | −31.7 (25.1) | 0.21 |
Model selected with all demographic factors in stepwise fashion, with the final model chosen by best Akaike Information Criterion. Effect estimates presented as with SE in parentheses.
OPO, organ procurement organization.
Compared with a model without the cluster groups, the model explained more of the variability between inpatient deaths among the OPOs (adjusted R2 0.86 versus 0.84), which suggested a better performance with clusters versus without. We also applied a percentage prediction error to the clustered model (the percentage difference between actual and predicted inpatient deaths), which identified 7 (12.5%) OPOs whose prediction was >10% different than actual inpatient deaths, indicating that the model could discriminate centers with different expected values of inpatient deaths as well.
DISCUSSION
The geographic variation in deceased organ donation reflects many factors, including underlying disease burden, quality of healthcare, population distribution, and resource availability.27 All contribute to a single output: the number of organ donors in a given OPO. To improve how OPOs are evaluated as targets for quality improvement, CMS has proposed using inpatient deaths as a better common denominator for the OPOs, rather than using eligible deaths. This number provides an objective assessment of donor potential within an OPO, but that number will vary between OPOs due to differing causes of death between the OPOs. Prior work has demonstrated a spatial relationship between the pattern of eligible deaths among the OPOs; we undertook this work to (1) assess this relationship among inpatient deaths and (2) demonstrate the role that geography and spatial associations play in the number of possible organ donors within an OPO and to understand how it could be used in future risk adjustment/performance assessment. In this article, we propose a method to assess spatial relationships of inpatient deaths between OPOs to fully capture the benefit of geographical data in this new metric.
We began by examining spatial associations between the eligible death rate and the inpatient death rate among the OPOs. In agreement with Cannon et al,18 we failed to find a geographic pattern in the eligible death rate (Figure 1A), but there was a significant geographic association related to the inpatient death rate (Figure 1B). This significant association prompted us to pursue further spatial analytic methods; if Moran’s I had not been indicative of a spatial relationship/component between inpatient deaths and geography, we would have otherwise proceeded with standard bivariate and multivariate analysis. We then measured the bivariate association patterns (Figure 2), which were ultimately similar to the patterns consistent with the regression analysis. This extension to bivariate spatial analysis is an important extension of prior work because it strengthens the cause for performing clustering and ultimately regression analyses.
Clustering was used to create cluster groupings based on the 5 most common causes of death for deceased organ donors and inpatient deaths. This identified a group of OPOs (Table 1 and Figure 3) with a greater rate of inpatient deaths (groups A and D), average rate (C), and lower rate (group B). The groupings were largely driven by the cause-specific death rates (Figure 3A), and identifying them as groups allows for further adjustment of the number of inpatient deaths and donor potential. Moreover, there were significant geographic associations in the race/ethnicity makeup, as well as the age distribution (Table 2).
After combining all analyzed factors into a multivariate model, the cluster groupings remained significantly associated with inpatient deaths (Table 3); in other words, even when the demographic characteristics and death rates are accounted for, a spatial relationship persists and helps to better explain the number of inpatient deaths. This analysis argues for including spatial relationships in the assessment of common OPO performance measures. Moreover, this shows which OPOs have more potential donors and therefore could be used to identify high- and low-performing OPOs to better understand and define best practices. These techniques can be extended to take advantage of more rich geographic data in the future, considering healthcare resource availability, differential patterns of infection and causes of death, and socioeconomic data.
There are limitations to the current work. Spatial statistics is subject to data quality and the ecological fallacy, as an individual may die in a hospital within an OPO’s DSA that differs from the OPO that covers the area inclusive of the decedent’s place of residence. Therefore, the dataset as utilized could misattribute death to the OPO where the decedent resides rather than where the decedent’s death actually took place (and therefore, where the opportunity for donation would have occurred). In the absence of other information, we would expect that effect to be relatively small among the 56 OPOs included in the study, although we note this could include deaths that occur while an individual is traveling as well as circumstances where a decedent lived on the border of 2 different OPO DSAs and underwent medical treatment in a different OPO’s service area than the decedent’s residence.
The present study intentionally does not assess the validity or usefulness of inpatient deaths as the denominator for OPO performance metrics, which would most likely be better assessed with direct patient-level characteristics that would not require other adjustments for OPO performance measurement. The process of converting inpatient deaths to an actual organ donor is complex, and therefore it is not particularly sensitive to all the parts of the donation process that affect the final outcome of donors and the organs recovered (eg, critical care, consent from donor or family, optimization of recoverable organs).28-30 This analysis cannot account for population growth and changes over time, nor can it assess for out of hospital deaths that may be appropriate for uncontrolled donation after circulatory death. Ultimately, we believe that these weaknesses are overcome because this approach is novel, is supported by prior work, and provides a method for more refined comparisons between OPOs.
In conclusion, we found a significant spatial/geographic component associated with inpatient deaths. This is to be interpreted in the context of the other contributing factors, namely the cause of death, age distribution, and racial/ethnic composition; these overlaps with the geographic component. Our analysis is timely and important because this modeling approach represents a method to assess policy and practice change by both the Organ Procurement and Transplantation Network and CMS. This method provides a useful platform for understanding geographic patterns among possible organ donors to drive quality improvement by improving the risk adjustment used to evaluate OPO performance. Ultimately, it is hoped that this will ultimately increase deceased donation and organ availability in the United States.
ACKNOWLEDGMENTS
The authors would like to thank H. Gilbert Welch, MD, for his insightful comments and critiques of the article.
Supplementary Material
Footnotes
Published online 11 February, 2021.
J.T.A. and T.D. both participated in the research design, writing of the article, performance of the research, and analysis of the results.
The authors declare no funding and conflicts of interest.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Scientific Registry of Transplant Recipients (SRTR). OPO-specific reports. Available at https://www.srtr.org/reports-tools/opo-specific-reports/. Accessed March 13, 2020.
- 2.Siminoff LA, Gardiner HM, Wilson-Genderson M, et al. How inaccurate metrics hide the true potential for organ donation in the United States. Prog Transplant. 2018; 28:12–18. [DOI] [PubMed] [Google Scholar]
- 3.Israni AK, Zaun D, Rosendale JD, et al. OPTN/SRTR 2017 annual data report: deceased organ donation. Am J Transplant. 2019; 19(Suppl 2):485–516. [DOI] [PubMed] [Google Scholar]
- 4.Haugen CE, Ishaque T, Sapirstein A, et al. Geographic disparities in liver supply/demand ratio within fixed-distance and fixed-population circles. Am J Transplant. 2019; 19:2044–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg D, Karp S, Shah MB, et al. Importance of incorporating standardized, verifiable, objective metrics of organ procurement organization performance into discussions about organ allocation. Am J Transplant. 2019; 19:2973–2978. [DOI] [PubMed] [Google Scholar]
- 6.Gentry SE, Chow EKH, Massie A, et al. Liver sharing and organ procurement organization performance. Liver Transpl. 2015; 21:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butala NM, King MD, Reitsma W, et al. Association between organ procurement organization social network centrality and kidney discard and transplant outcomes. Transplantation. 2015; 99:2617–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The White House. Executive order on advancing American kidney health. 2019. Available at https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/. Accessed March 13, 2020.
- 9.Centers for Medicare & Medicaid Services (CMS.gov). Organ Procurement Organization (OPO) conditions for coverage proposed rule: revisions to outcome measures for OPOs. 2019. Available at https://www.cms.gov/newsroom/fact-sheets/organ-procurement-organization-opo-conditions-coverage-proposed-rule-revisions-outcome-measures-opos. Accessed April 19, 2020.
- 10.Tonelli M, Klarenbach S, Rose C, et al. Access to kidney transplantation among remote- and rural-dwelling patients with kidney failure in the United States. JAMA. 2009; 301:1681–1690. [DOI] [PubMed] [Google Scholar]
- 11.Axelrod DA, Guidinger MK, Finlayson S, et al. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008; 299:202–207. [DOI] [PubMed] [Google Scholar]
- 12.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010; 5:2276–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler JT, Yeh H, Markmann JF, et al. Market competition and density in liver transplantation: relationship to volume and outcomes. J Am Coll Surg. 2015; 221:524–531. [DOI] [PubMed] [Google Scholar]
- 14.Adler JT, Yeh H, Markmann JF, et al. Temporal analysis of market competition and density in renal transplantation volume and outcome. Transplantation. 2016; 100:670–677. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee P, Venkataramani AS, Vijayan A, et al. The effect of state policies on organ donation and transplantation in the United States. JAMA Intern Med. 2015; 175:1323. [DOI] [PubMed] [Google Scholar]
- 16.Reibel M, Olmo C, Andrada S, et al. Deep demographics: understanding local variation in donor registration. Prog Transplant. 2016; 26:191–198. [DOI] [PubMed] [Google Scholar]
- 17.Ladin K, Wang R, Fleishman A, et al. Does social capital explain community-level differences in organ donor designation? Social capital and community differences in organ donor designation. Milbank Q. 2015; 93:609–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon RM, Jones CM, Davis EG, et al. Patterns of geographic variability in mortality and eligible deaths between organ procurement organizations. Am J Transplant. 2019; 19:2756–2763. [DOI] [PubMed] [Google Scholar]
- 19.Tobler W. A computer movie simulating urban growth in the Detroit region. Econ Geogr. 1970; 45:234–240. [Google Scholar]
- 20.Centers for Disease Control and Prevention. CDC WONDER. Available at https://wonder.cdc.gov/. Accessed March 16, 2020.
- 21.Snyder JJ, Musgrove D, Zaun D, et al. The Centers for Medicare and Medicaid Services’ proposed metrics for recertification of organ procurement organizations: evaluation by the scientific registry of transplant recipients. Am J Transplant. 2020; 20:2466–2480. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y. New approaches for calculating Moran’s index of spatial autocorrelation. PLoS One. 2013; 8:e68336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anselin L, Syabri I, Smirnow O. Visualizing multivariate spatial correlation with dynamically linked windows. In: New Tools for Spatial Data Analysis: Proceedings of the Specialist Meeting, BioMedware Conference on Space-Time Information Systems, Ann Arbor, MI, January 11–12, 2002, and the Annual Meetings of the Association of American Geographers, Los Angeles, CA, March 2002. [Google Scholar]
- 24.Chavent M, Kuentz-Simonet V, Labenne A, et al. ClustGeo: an R package for hierarchical clustering with spatial constraints. Comput Stat. 2018; 33:1799–1822. [Google Scholar]
- 25.Anselin L, Syabri I, Kho Y. GeoDa: an introduction to spatial data analysis. Geogr Anal. 2006;; 38:5–22. [Google Scholar]
- 26.Adler JT. Replication data for: evaluating spatial associations in inpatient deaths between organ procurement organizations. [Epub ahead of print 2020]. doi: 10.7910/DVN/J1A4KS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch RJ, Patzer RE. Geographic inequity in transplant access. Curr Opin Organ Transplant. 2019; 24:337–342. [DOI] [PubMed] [Google Scholar]
- 28.Luskin R, Nathan H. Eligible death statistic: not a true measure of OPO performance nor the potential to increase transplantation. Am J Transplant. 2015; 15:2019–2020. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg DS, Shafer T, Siminoff L. Important facts about organ donation and OPO performance. Transplantation. 2018; 102:e249–e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croome KP, Lee DD, Keaveny AP, et al. Noneligible donors as a strategy to decrease the organ shortage. Am J Transplant. 2017; 17:1649–1655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


