Table 1.
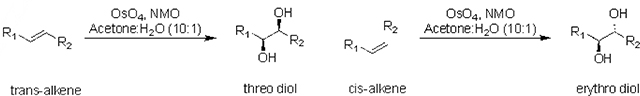
The binding constants, KH:G, determined through UV-Vis titration and Os-reaction scheme for the threo and erythro diols.
| Set | trans-alkene | cis-alkene | threo diol | erythro diol |
|---|---|---|---|---|
| 1: R1, R2 = CH3 | G1T | G1C | G1TH | G1ER |
| KH:G[a] | — | — | 0.348 | 0.057 |
| 2: R1, R2 = Ph | G2T | G2C | G2TH | G2ER |
| KH:G[a] | — | — | 11.1 | [b] |
| 3: R1 = (CH2)7CH3 R2 = (CH2)7COOCH3 |
G3T | G3C | G3TH | G3ER |
| KH:G[a] | — | — | 1.38 | [b] |
| 4: R1, R2 = (CH2)2CH3 | G4T | G4C | G4TH | G4ER |
| KH:G[a] | — | — | 1.10 | 0.16 |
| 5: R1 = CH3 R2 = (CH2)2CH3 |
G5T | G5C | G5TH | G5ER |
| KH:G[a] | — | — | 2.64[c] | 0.29[c] |
| 6: R1, R2 = CH(CH3)2 | G6T | G6C | G6TH | G6ER |
| KH:G[a] | — | — | 3.72 | [b] |
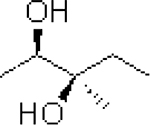
| 7: | 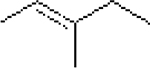 |
 |
 |
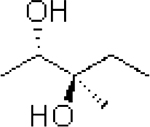 |
| G7T | G7C | G7TH | G7ER | |
| KH:G[a] | — | — | 1.41[c] | 0.41 [c] |
 | ||||
103 M−1
negligible measurement
binding constants determined using the 96-well plate, see supplementary information.
