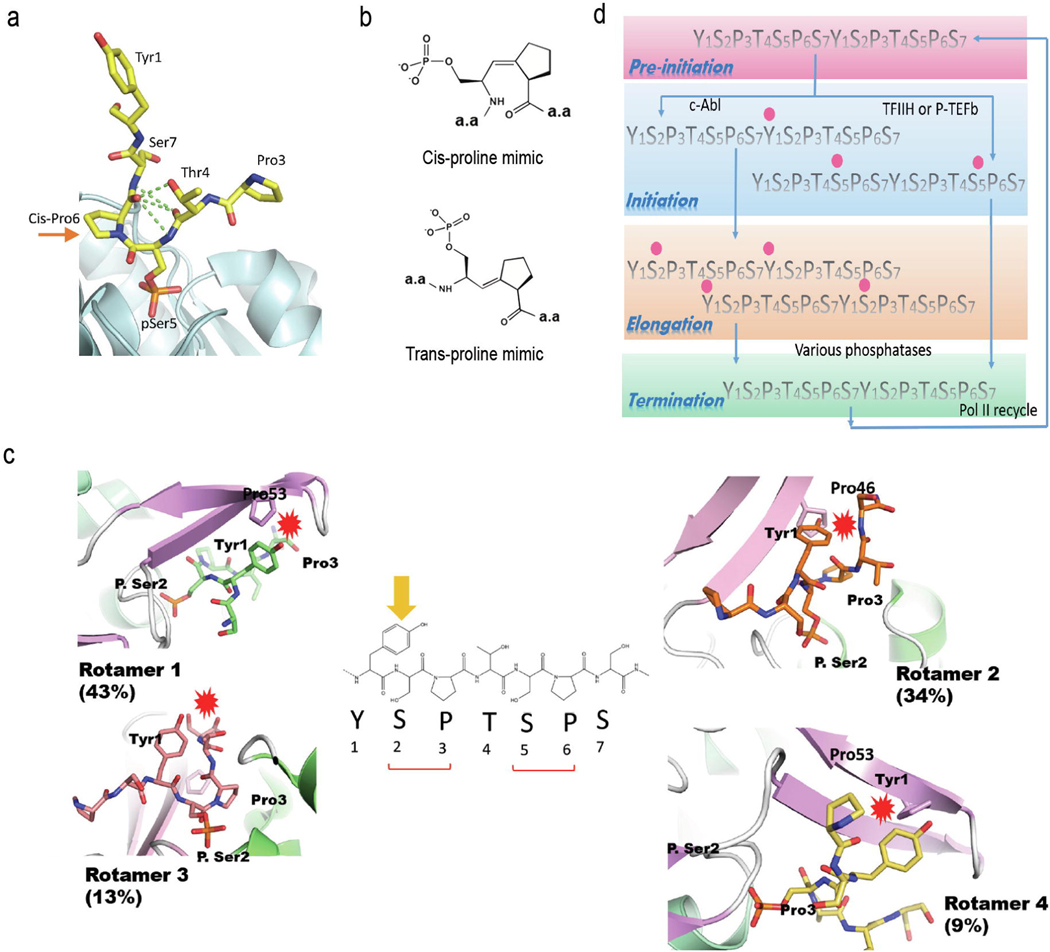
Figure 2:
Ssu72 dephosphorylates Ser5 of the CTD with the requirement of Pro6 is in the cis configuration. (a) The complex structures of Ssu72 bound to its substrates reveal that the proline residue is always in the cis configuration next to the dephosphorylation sites. (b) Chemical structures of the Isostere homologs mimic proline residues locked in cis or trans configurations. (c) Ssu72 has little activity towards Ser2 of the CTD because the flanking residues would cause steric clashes. (d) Crosstalk of Tyr1 and Ser2 phosphorylation leads to differentiated outcomes in transcription. Tyr1 phosphorylation primes the P-TEFb mediated Ser2 phosphorylation to promote elongation.