Figure 7.
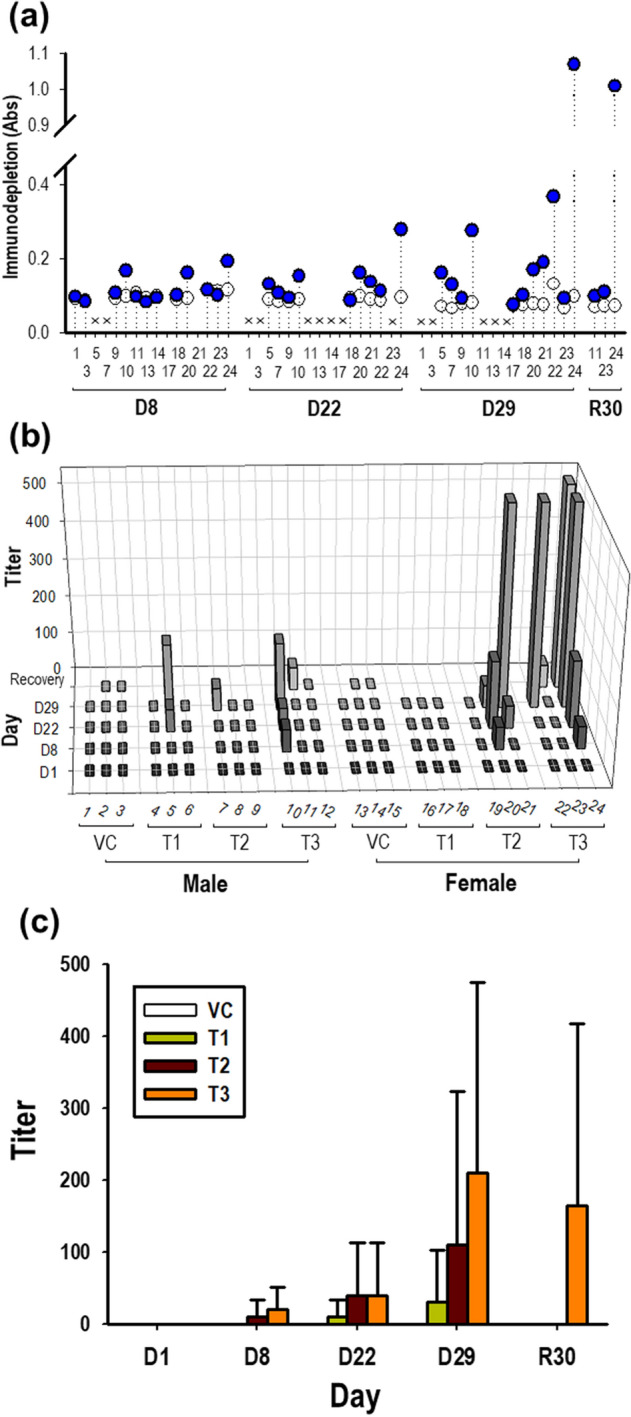
Measurement of ADAs, representing anti-GX-G3 antibodies, in monkeys subjected to 4-week repeated subcutaneous injection with GX-G3 followed by a 4-week recovery period. Monkeys were separated into 4 injection subgroups: VC (vehicle control), T1 (1 mg/kg GX-G3), T2 (3 mg/kg GX-G3), and T3 (10 mg/kg GX-G3). ADA levels in monkey sera were measured using the method validated in the present study. (a) Immuno-competition assays were performed to discriminate between true and false positive samples. True positivity for GX-G3 specificity was identified based on a greater than 30% difference 30% in absorbance between the non-treated and GX-G3-treated samples. (b) Monkey serum samples determined to be true positives were prepared as serial three-fold dilutions beginning from 20-fold. The antibody titer of each sample was expressed as the highest fold-dilution displaying a positive response (absorbance value > normalized NCO value). (c) The mean antibody titer of each group was arithmetically calculated. VC, vehicle control group; D1, D8, D22 and D29, dosing days 1, 8, 22 and 29, respectively; R30, recovery day 30.
