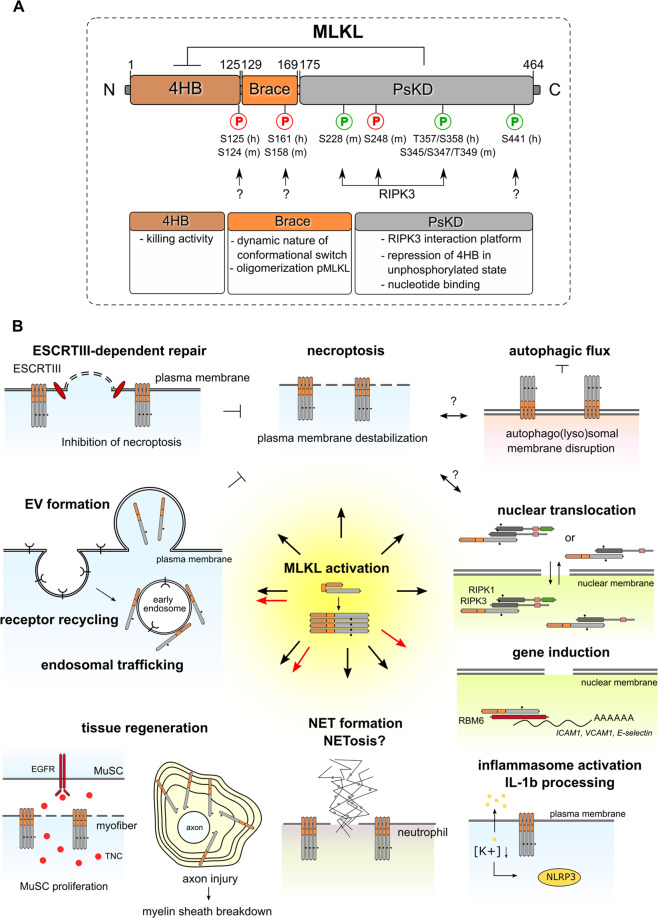
Fig. 2. Structural domains of MLKL and their functions.
A The functions of each structural domain of MLKL are indicated. The N-terminal 4-helical bundle domain (4HB) shows homology with the N-terminal HeLo-like domain (HELL) of the fungal protein HELLP according to Hidden Markov Models [128]. The PsKD resembles a bi-lobal protein kinase domain, which binds ATP without hydrolyzing it, thus rendering the pseudokinase domain catalytically inactive [91]. Upon RIPK3-dependent phosphorylation (S345) of MLKL, the self-inhibitory pseudokinase domain (PsKD) of MLKL is released from the 4HB, thereby activating MLKL (pMLKLS345) to induce necroptosis. The brace region contributes to the dynamic nature of the 4HB domain, the conformational change induced after activating phosphorylation of the PsKD and the oligomerization of activated MLKL. Phosphorylations that tune cell death activity of MLKL include inhibitory phosphorylation and activating phosphorylation (indicated in red and green respectively). Impact of the phosphorylation of MLKL on its cell death-independent functions is not known yet. h human site, m murine site. B Next to necroptosis execution, MLKL is also involved in ESCRT (Endosomal Sorting Complexes Required for Transport)-dependent repair of the plasma membrane (which restricts necroptotic cell death), release of extracellular vesicles, endosomal trafficking and receptor recycling, myelin sheath membrane breakdown and axon regeneration after injury, muscle stem cell (MuSC) proliferation after muscle injury, NET formation, inflammasome activation, possible nuclear functions including regulation of endothelial cell adhesion molecules such as ICAM1, VCAM1 and E-selectin through interaction with RNA-binding motif protein 6 (RBM6) and stabilization of mRNA, and inhibition of autophagic flux. TNC: tenascin-C. Black dot: phosphorylation of MLKL. Black arrows: processes that require RIPK3-dependent phosphorylation of MLKL, while red arrows: processes that require RIPK3-independent phosphorylation of MLKL.