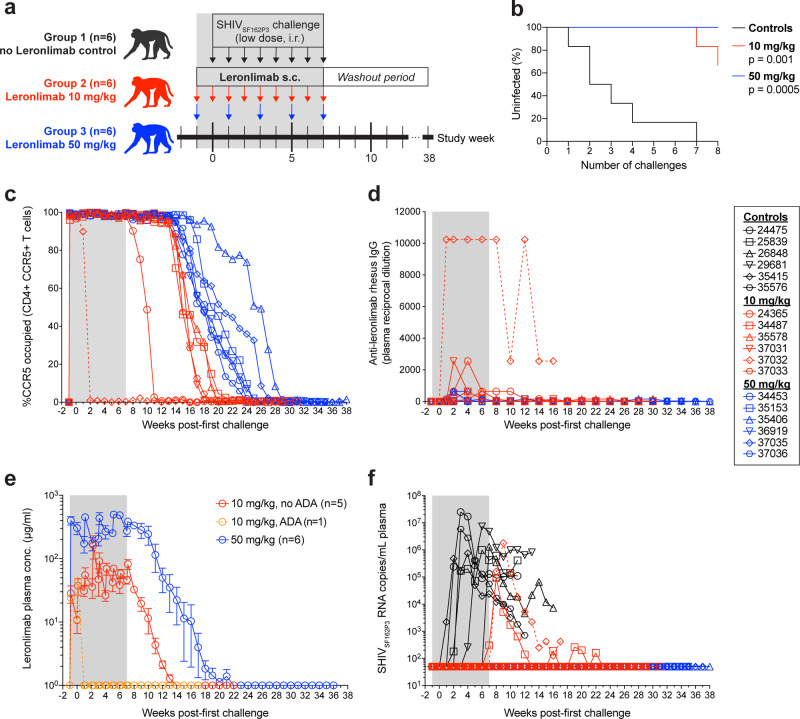
Fig. 2. Leronlimab pre-exposure prophylaxis protects rhesus macaques from intrarectal SHIVSF162P3 acquisition.
a Study outline. Eighteen rhesus macaques were challenged with 3.2 TCID50 of SHIVSF162P3 weekly via intrarectal inoculation for 8 consecutive weeks. Group 1 received no Leronlimab (black, n = 6) while Group 2 received weekly 10 mg/kg Leronlimab (red, n = 6) and Group 3 received biweekly or once-every-two-weeks Leronlimab (blue, n = 6). b Kaplan–Meier curve comparing the percentage of uninfected to the number of SHIVSF162P3 challenges, analyzed by log-rank test with Dunn’s correction. c Longitudinal CCR5 receptor occupancy levels on peripheral blood CCR5+ CD4+ T cells in Leronlimab-treated macaques as determined by flow cytometry (see “Methods”). d Longitudinal anti-Leronlimab rhesus IgG levels in plasma of Leronlimab-treated macaques. e Mean (±SEM) longitudinal Leronlimab plasma concentrations in Groups 2 and 3, separated by development of persistent anti-Leronlimab rhesus IgG antibody levels (ADA antidrug antibodies; orange). f Longitudinal SHIVSF162P3 plasma viral loads. Horizontal dashed line denotes assay limit of quantification (50 copies/mL); gray boxes in a, c–f denote the Leronlimab treatment phase for Leronlimab-treated macaques. Legend at right shows symbols used in panels c, d, f identifying individual rhesus macaques. The macaque that developed persistent anti-Leronlimab rhesus IgG antibody levels (37032) is shown as a dashed line throughout panels c–f. Group colors and individual animal symbols are consistent throughout the manuscript. Source data are provided as a Source Data file.